OK, click baity title, but bear with me. In a prior post on Roman fertility decline, I had mentioned a speculative piece arguing that Roman fertility decline was in part due to their passion for hot showers. Seems far fetched, but maybe it is not so far fetched. Consider this study that’s been floating around:
- Garolla, A., Torino, M., Sartini, B., Cosci, I., Patassini, C., Carraro, U., & Foresta, C. (2013). Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Human Reproduction, 28(4), 877-885. PDF
Study question: What are the effects of continuous sauna exposure on seminal parameters, sperm chromatin, sperm apoptosis and expression of genes involved in heat stress and hypoxia?
Summary answer: Scrotal hyperthermia by exposure to sauna can induce a significant alteration of spermatogenesis.
What is known already: Several authors have evidenced that high temperature has dramatic effects on spermatogenesis.
Study design, size and duration: A longitudinal time-course study. Data from 10 subjects exposed to Finnish sauna were collected before sauna (T0), after 3 months of sauna sessions (T1) and after 3 (T2) and 6 months (T3) from the end of sauna exposure.
Participants/materials, setting and methods: Ten [10] normozoospermic [normal quality spermers] volunteers underwent two sauna sessions per week for 3 months, at 80-90°C, each lasting 15 min. Sex hormones, sperm parameters, sperm chromatin structure, sperm apoptosis and expression of genes involved in heat stress and hypoxia were evaluated at the start, at the end of sauna exposure and after 3 and 6 months from sauna discontinuation. Student’s t-test for paired data was used for statistical analysis.
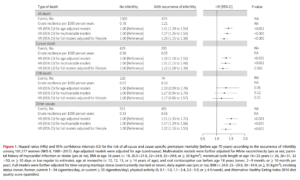
Main results and the role of chance: At the end of sauna exposure, we found a strong impairment of sperm count and motility (P < 0.001), while no significant change in sex hormones was present. Decreases in the percentage of sperm with normal histone-protamine substitution (78.7 ± 4.5 versus 69.0 ± 4.1), chromatin condensation (70.7 ± 4.7 versus 63.6 ± 3.3) and mitochondrial function (76.8 ± 4.9 versus 54.0 ± 6.1) were also evident at T1, and strong parallel up-regulation of genes involved in response to heat stress and hypoxia was found. All these effects were completely reversed at T3.
Limitations and reasons for caution: Absence of subjects with abnormal sperm parameters was the major limitation of this study.
Wider implications of the findings: Our data demonstrated for the first time that in normozoospermic subjects, sauna exposure induces a significant but reversible impairment of spermatogenesis, including alteration of sperm parameters, mitochondrial function and sperm DNA packaging. The large use of Finnish sauna in Nordic countries and its growing use in other parts of the world make it important to consider the impact of this lifestyle choice on men’s fertility.
Study funding/competing interest(s): No external funding was sought for this study and the authors have no conflict of interest to declare.
Contrary to most science writing, the abstract quite undersells the study. They had 10 people with no sperm issues, and then gave them Finnish saunas twice a week for 3 months. It looks like this:

Notice the times. T2 is measured 3 months after exposure to sauna but it still shows effects of sauna for total sperm count! The effect after 3 months of going to sauna 2 times a week is remarkable. The decline is more than 50% of sperm concentration/count.
The problem here is that this study has 10 people in it and no control group. The p values do not seem hacked. I looked for a replication. Fortunately, this study is well-cited, giving me 145 citing articles to check. And there is this Chinese replication:
- Zhang, M. H., Zhang, A. D., Shi, Z. D., Wang, L. G., & Qiu, Y. (2015). Changes in levels of seminal nitric oxide synthase, macrophage migration inhibitory factor, sperm DNA integrity and caspase-3 in fertile men after scrotal heat stress. PLoS One, 10(10), e0141320.
Background This study observes changes in levels of seminal nitric oxide (NO), nitric oxide synthase (NOS), macrophage migration inhibitory factor (MIF), sperm DNA integrity, chromatin condensation and Caspase-3in adult healthy men after scrotal heat stress (SHS).
Methods Exposure of the scrotum of 25 healthy male volunteers locally at 40–43°C SHS belt warming 40 min each day for successive 2 d per week. The course of SHS was continuously 3 months. Routine semen analysis, hypo-osmotic swelling (HOS) test, Aniline blue (AB) staining, HOS/AB and terminal deoxynucleotidyl transferase-mediated d UDP nick-end labeling (TUNEL) were carried out before, during and after SHS. Seminal NO and NOS contents were determined by nitrate reduction method. The activated Caspase-3 levels of spermatozoa and MIF in seminal plasma were measured by the enzyme-linked immunosorbent assay (ELISA) method. Statistical significance between mean values was determined using statistical ANOVA tests.
Results The mean parameters of sperm concentration, motile and progressive motile sperm and normal morphological sperm were significantly decreased in groups during SHS 1, 2 and 3 months compared with those in groups of pre-SHS (P<0.001). Statistically significant differences of sperm DNA fragmentation, normal sperm membrane, and Caspase-3 activity as well as the level of NO, NOS and MIF in semen were observed between the groups before SHS and after SHS 3 months and the groups during SHS 1, 2 and 3 months (P<0.001). After three months of the SHS, various parameters recovered to the level before SHS. WBC in semen showed a positively significant correlation with the levels of NO, NOS, MIF and Caspase-3 activity. The percentage of abnormal sperm by using the test of HOS showed a positively significant correlation with that of HOS/AB.
Conclusions The continuously constant SHS can impact the semen quality and sperm DNA and chromatin, which may be contributed to the high level of NO, NOS, MIF and Caspase-3 by SHS.
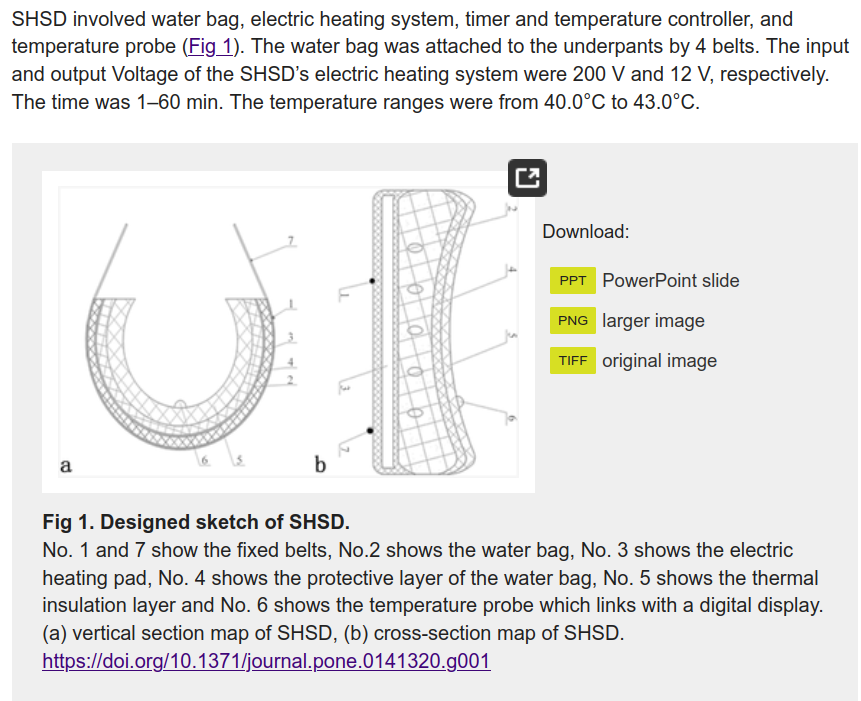
So this time it is 25 Chinese people who were given “scrotal heat stress” (SHS), which has to be the funniest euphemism you have read in a while. Anyway, their subjects were heated up for 40 minutes, 2 days a week for 3 months. I wish they had included a photo of their “Scrotal Heat Stress Device”. Unfortunately, we are stuck making sense of this diagram:
Their results looks like this:
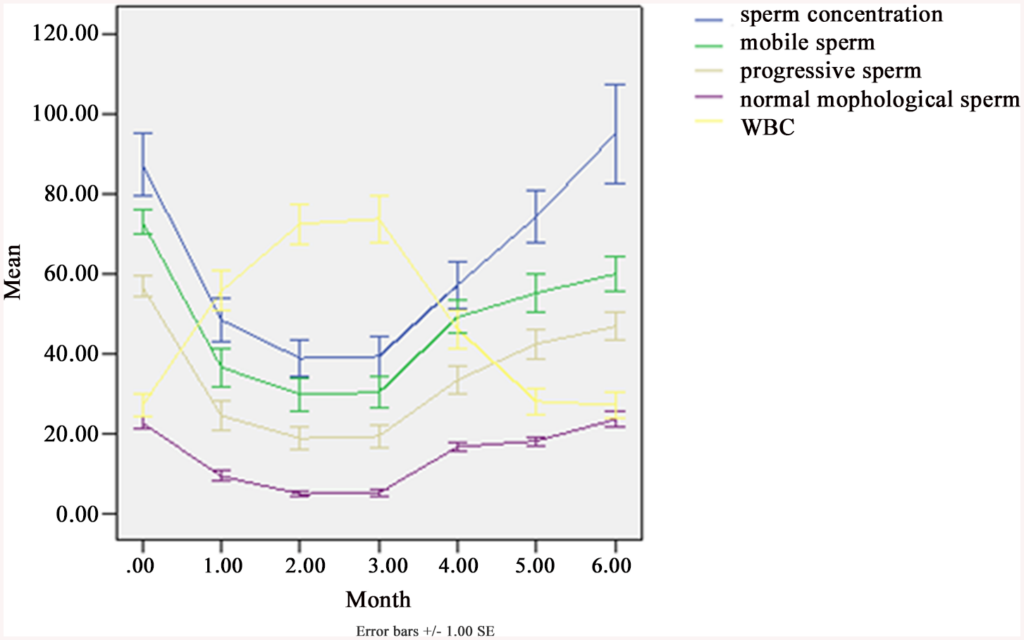
Fig 3. Sperm concentration, progressive motility (grade “a” + “b”), motile sperm, morphologically normal sperm and white blood cells (WBC) in semen, before, during and after SHS in 25 subjects.
Concentration (× 106/ml), Motile sperm = grade (“a” + “b” + “c”)%, Progressive = grade (“a” + “b”)%, Normal morphological sperm (%), WBC (× 106/ml). From SPSS 13.0. Mean ± SEM.
The treatment ran 3 moths, so that’s the data from 0 (just before treatment) and until 3 months. 1 month after treatment (4 months on x axis), we see that most values are still reduced, and this is also true for 2 months post treatment (5 months on plot). 3 months after treatment (6 months on plot) some are still below (progressive sperm, mobile sperm) but others have returned to normal (sperm concentration, white block cells, normal sperm %). So this study finds that the effects last at least 2 months, and maybe 3 months or more.
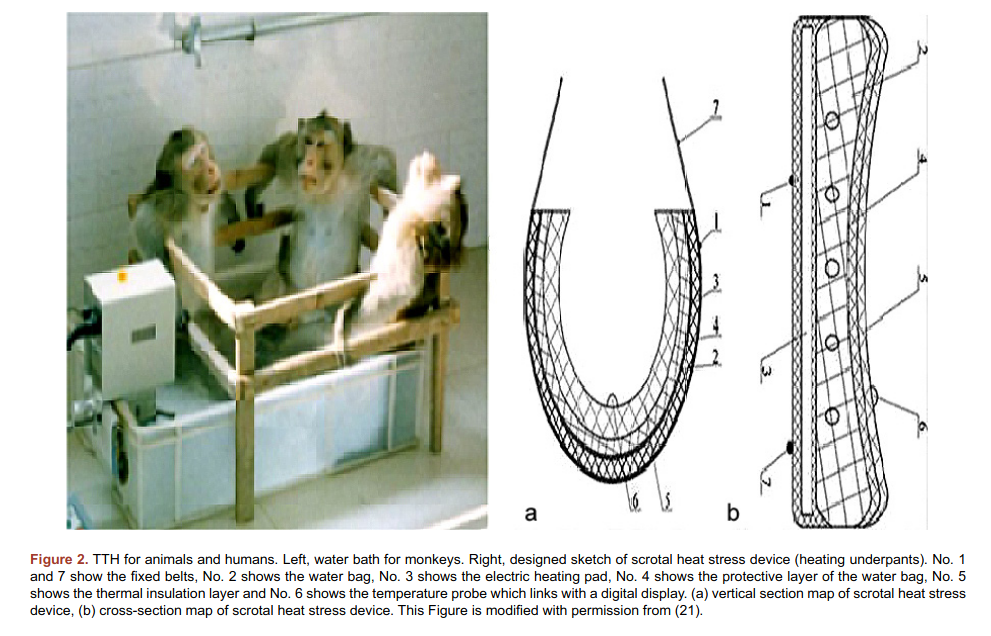
The main caveat of this study is that the authors published it twice thrice, and in the first version, February 2015, there are only 19 persons in the study, and then there’s 25. So what gives? Maybe it isn’t too fishy, but then there’s this 2018 study by the same authors, and now the study had 30 persons! Oh and by the way, the same authors published a review of these studies in 2019, which included a photo of the method used for treatment of monkeys.
OK, so maybe we don’t actually trust that study, but is there a less fraudulent looking replication? Yes, another Chinese study:
- Rao, M., Zhao, X. L., Yang, J., Hu, S. F., Lei, H., Xia, W., & Zhu, C. H. (2015). Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian journal of andrology, 17(4), 668.
This prospective randomized clinical study is aimed to evidence the reproductive impairment of frequent scrotal heat exposure. A total of 20 normozoospermic subjects were randomly divided into two groups to undergo testicular warming in a 43 °C water bath 10 times, for 30 min each time; the subjects in group 1 underwent testicular warming for 10 consecutive days and those in group 2 once every 3 days. Sperm chromatin structure assay (SCSA), sperm mitochondrial membrane potential (MMP), apoptosis, and seminal plasma-soluble Fas (sFas) were analyzed before treatment and every 2 weeks after, for a total of 10 times. In group 1, some critical proteins involved in heat stress, hypoxia, structure, and function of sperm mitochondria and flagella were evaluated before hyperthermia and 2, 6, 10, and 16 weeks after hyperthermia. Both groups showed a reversible increase in the proportion of spermatozoa with a disrupted MMP (both p < 0.05 when the minimums were compared with baseline levels, the same below), sperm apoptosis (both p < 0.01) and high DNA stainability (both p < 0.05). The sFas concentration in both groups showed no obvious changes except one: the value at week 2 was significantly increased over baseline in group 1 (p = 0.036). The level of Bcl-2 decreased significantly at weeks 6 and 10 (p = 0.017 and 0.05, respectively) and recovered to baseline at week 16. Proteins involved in heat stress and mitochondria functions were up-regulated, whereas in flagella structure and function was down-regulated (all p < 0.05). This study demonstrated that transient and frequent scrotal hyperthermia severely and reversibly damaged spermatogenesis, consecutive heat exposure had more serious effects than intermittent exposure, whereas intermittent exposure led to a later recovery of sperm damage.
As far as I can tell, the authors do not overlap with the other 2015 study. They had 20 subjects, which they randomized into treatment and more treatment. The subjects were given 10 warm baths in 43° water (slightly warmer than body temperature). The more-treatment group was given this 10 days in a row, and the less-treatment group only every 3rd day. So the treatment for the more-treatment group lasted about 30 days. The results:
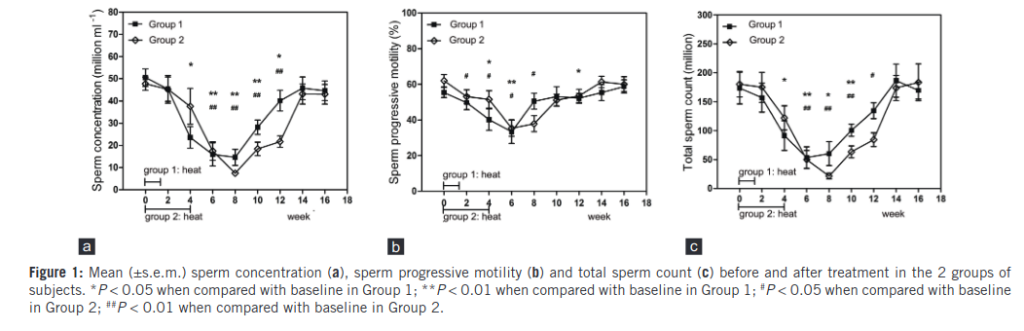
The shape of these plots is by now familiar. There is a steep decline, followed by a long recovery period. We see that the more-treatment group declines faster, and also rebounds slightly faster. But only at weak 14, that is 88 days after treatment, do they reach their pre-treatment levels. That’s about the 3 month mark we saw in the other studies.
The Rao study was also double published, the second study in 2016. BUT at least the number of subjects stayed the same, so this is not so suspicious. For the non-researchers reading this and wondering, “why do researchers bother to write-up studies multiple times? doesn’t that waste a lot of time?”. It does, but academia is a game about maximizing published papers and citations to them. Collecting data and doing studies takes more time (and money) than writing up the same study in different ways and publishing it in different journals. Or just splitting the content up and publishing it in parts, called salami publishing.
There may be more studies, I stopped looking after finding 2 replications. At this point, I feel pretty confident that heat stress has quite long lasting effects on semen quality. Why do we care? Well, human sperm counts are decreasing:
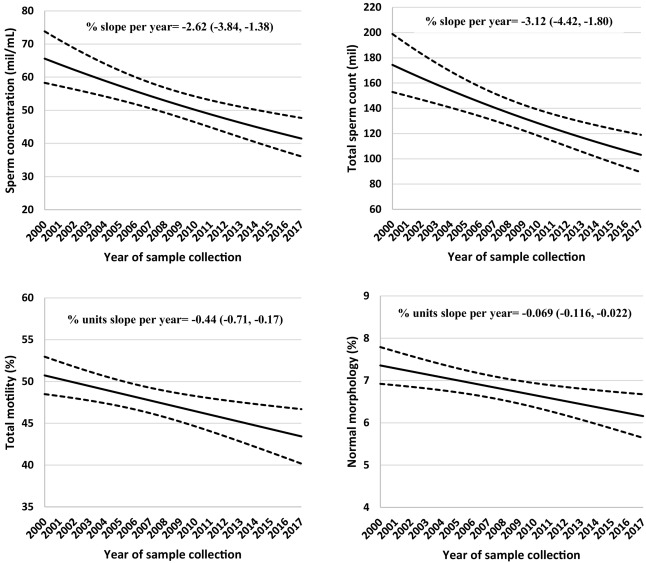
Fig. 1. Secular trends in sperm concentration, total sperm count, sperm motility and normal morphology among men attending a fertility center in Boston between 2000 and 2017. The solid lines represent the adjusted mean for semen parameters by year of sample collection, the dotted lines are the upper and lower 95% confidence intervals for the adjusted means. Adjusted means are presented for average abstinence time (2 days). N = 936 men and 1618 semen samples for all parameters except sperm morphology, which was assessed in 1482 semen samples.
These decreases are seen in many countries, and there’s more than one meta-analysis of the topic. This 2018 may be the latest one, the 2017 one is more famous. Interestingly, a 2020 meta-analysis of 33 studies of ball size did not find any secular change. So it seems that we have something causing a decline in sperm quality/count, despite positive selection for this trait, and it’s not related to ball size. So what gives? Too much work sitting down? Should men resume wearing dresses? Too many people with laptops on their thighs? Too tight pants? Too much time spent in-doors in higher, more comfortable temperatures? Some of the decline is surely also due to obesity, which seems to itself cause temperature issues due to reduced airflow. All of these seem true to some extent, but causality is harder than noting temporal patterns that fit. Whatever the cause of the historical decline in sperm quality, the evidence says you should avoid saunas and hot pools if you are trying to become a dad.