
- David A. Sinclair – Lifespan: Why We Age—and Why We Don’t Have To, 2019 (free ebook is on libgen)
I know Scott Alexander has already reviewed this, but since I kind of make a habit of reading books he reviewed favorably, and this book overlapped with my general interest in biotech, I decided to give it a go. I’ve been working in biotech in a professional capacity for about 7 years, though not much in the anti-aging field, mainly in genomics.
The book’s table of contents looks like this:
- WHAT WE KNOW
- Chapter 1. VIVA PRIMORDIUM
- Chapter 2. THE DEMENTED PIANIST
- Chapter 3. THE BLIND EPIDEMIC
- WHAT WE’RE LEARNING
- LONGEVITY NOW
- A BETTER PILL TO SWALLOW
- BIG STEPS AHEAD
- THE AGE OF INNOVATION
- WHERE WE’RE GOING
- THE SHAPE OF THINGS TO COME
- A PATH FORWARD
- CONCLUSION
So there’s 3 parts with the majority of the chapters, followed by the conclusion. The most interesting part to me was the scientific parts. The other parts are about changing the narrative and considering aging a disease, so anti-aging research can get more funding, and people stop just blindly accepting death as unavoidable (“it was his time to go”). If you are a typical grey tribe reader, nothing of this will be new to you, probably even dull due to his many repeats (“yes, I already want to stop aging, I get it aging bad!, can we please move on??”). The latter parts speculates about issues this will cause, and especially how to reconcile more old people with his woke ideas (yes, yikes!). Scott’s review cover the science ideas in detail, I am going to focus on the credibility from a statistics and source perspective.
Whenever you read anything on health matters, be ultra-skeptical. Everybody has their own pet theories in health. Considering the scientific quality of that kind of research, and the constant diet fads, your prior should be very skeptical indeed on any claim. So when Sinclair repeatedly reports us rather fantastic results from animal studies on say calorie restriction or body cooling, let’s have a closer look at these. Are they going to be the usual n=12 mice, p = .04 ordeals? Let’s find out!
While reading, I marked some passages for comment. Later I just picked random parts where he makes some claim with reference to some study.
We are making progress every week in restoring the youthful epigenome of mice by delivering reprogramming factors. The pace of discovery is mind spinning. A full night of sleep for me and my lab members is increasingly rare.
In the 1990s, there were major concerns about the safety of delivering genes to humans. But there are a rapidly increasing number of approved gene therapy products and hundreds of clinical trials under way. Patients with an RPE65 mutation that causes blindness, for example, can now be cured with a simple injection of a safe virus that infects the retina and delivers, forever, the functional RPE65 gene.
Sounds fantastic but no source is given that I can find. OK, so I searched Scholar and … it checks out:
- Pierce, E. A., & Bennett, J. (2015). The status of RPE65 gene therapy trials: safety and efficacy. Cold Spring Harbor perspectives in medicine, 5(9), a017285.
- Trapani, I., & Auricchio, A. (2018). Seeing the light after 25 years of retinal gene therapy. Trends in molecular medicine, 24(8), 669-681.
This one is pretty cool, somehow I hadn’t heard of it. There’s a 2020 general review of gene therapy via adeno viral vectors.
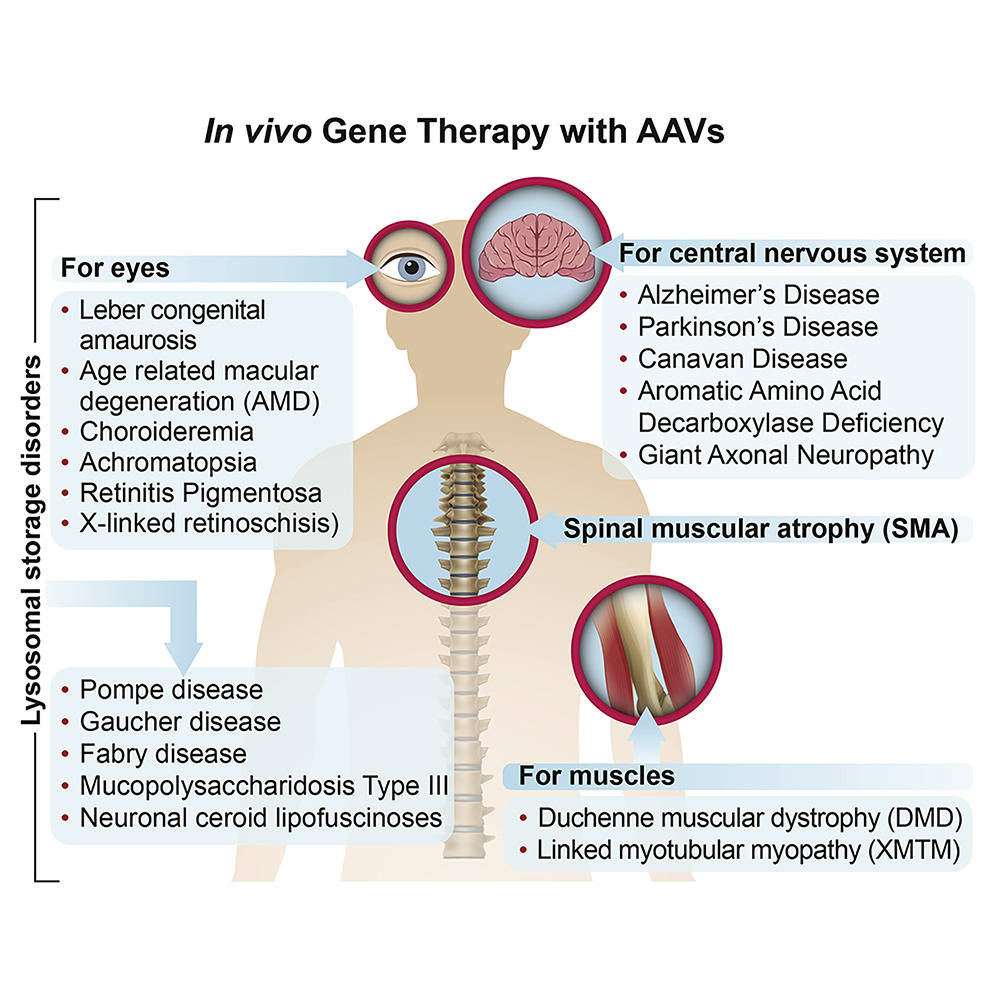
Next claim:
If females and males are in the same environment, in general, females will live longer. It’s a common theme throughout the animal kingdom. Scientists have tested whether it is the X chromosome or the ovary that is important. Using a genetic trick, they created mice with one or two Xs, with either ovaries or testes.9 Those with a double dose of the X lived longer, even if they had testes and especially if they didn’t, thus proving once and for all that female is the stronger sex.
Source goes to:
- E. J. Davis, I. Lobach, and D. B. Dubal, “Female XX Sex Chromosomes Increase Survival and Extend Lifespan in Aging Mice,” Aging Cell 18, no. 1 (February 2019), e12871, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351820/.
Female longevity is observed in humans and much of the animal kingdom, but its causes remain elusive. Using a genetic manipulation that generates XX and XY mice, each with either ovaries or testes, we show that the female XX sex chromosome complement increases survival during aging in male and female mice. In combination with ovaries, it also extends lifespan. Understanding causes of sex‐based differences in aging could lead to new pathways to counter age‐induced decline in both sexes.
Their results:
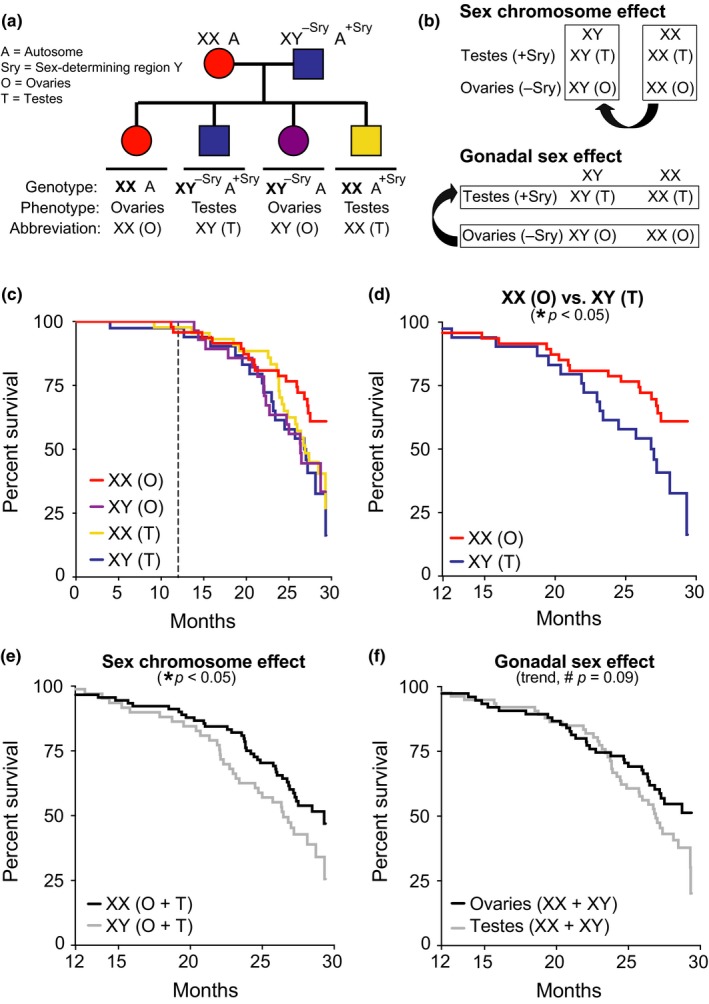
XX sex chromosomes contribute to female longevity. (a) Diagram of FCG model. XX females were crossed with XY males with the Sry on an autosome instead of the Y chromosome. (b) Strategy to identify causes of sexual dimorphism using the FCG model by testing main effect of sex chromosomes (top) and main effect of gonads (bottom). (c–f) Kaplan–Meier curves of FCG aging cohort (n = 261 mice): XX(O) n = 64, XY(T) n = 48, XX(T) n = 94, and XY(O) n = 55. (c) In all groups, survival was tracked until 30 months and statistical analyses were performed with left censoring prior to 12 months as indicated by dotted vertical line. (d) Stratified pairwise hazard model comparisons show that XX(O) mice exhibit less mortality than XY(T) mice (XX(O), HR = 0.45, CI = 0.23–0.88, *p = 0.02). Cox proportional hazard model analysis shows (e) main effect of sex chromosome complement (XX, HR = 0.60, CI = 0.37–0.96, *p = 0.03) and (f) trend in gonadal effect (ovaries, HR = 0.66, CI = 0.41–1.06, #p = 0.09). HR = hazard ratio and CI = confidence interval; HR < 1 is decreased mortality risk (statistical details in Supporting Information Tables S1 and S2)
Every p value is bad! This is shit tier even if we accept the experimental setup.
The future is bright due to early detection using genomics and biomarkers:
There are more than a hundred companies just in the United States pursuing lightning-fast, superfocused DNA testing that can offer us early and accurate diagnoses of a vast range of ailments and even estimate our rate of biological aging.18 A few are aimed at detecting the genetic signature of cancer and other illnesses years before they can normally be detected. Soon, we will no longer have to wait for tumors to grow so big and so heterogeneously mutated that their spread is no longer controllable. With a simple blood test, doctors will be able to scan for circulating cell-free DNA, or cfDNA, and diagnose cancers that would be impossible to spot without the aid of computer algorithms optimized by machine learning processes trained on thousands of cancer patient samples. These circulating genetic clues will tell you not just if you have cancer but what kind of cancer you have and how to kill it. They will even tell you where in your body an otherwise undetectable tumor is growing, since the genetic (and epigenetic) signatures of tumors in one part of the body can be vastly different from those from other parts.19
All of this means we’re on the way to a fundamental shift in the way we search for, diagnose, and treat disease. Our flawed, symptom-first approach to medicine is about to change. We’re going to get ahead of symptoms. Way ahead. We’re even going to get ahead of “feeling bad.” Many diseases, after all, are genetically detectable long before they are symptomatic. In the very near future, proactive personal DNA scanning is going to be as routine as brushing our teeth. Doctors will find themselves saying the words “I just wish we’d caught this earlier” less and less—and eventually not at all.
But the coming age of genomics is just the start.
I agree that faster detection of issues is a key way forward for combating aging. While sometimes we cannot do much or anything about despite early detection (e.g. dementia), for cancers, early detection is key to survival:

Basically, if we did personalized bloodstream based sequencing/biomarker testing every year or so, we could probably catch ~all cancers in stage 1. This would add something like 5 years of lifespan (really? yes, because once you start getting cancer, you will also start dying of other stuff, so the net effect is not so large).
Along the way, I am creating data specific to my body. And over time, that data is helping me identify negative and positive trends that may be subtly different for me than for other people. We know, of course, that our genetic heritage can have a significant impact on the sorts of food our bodies need, tolerate, or reject, but everyone’s genetic heritage is different. What you need, what your partner needs, and what your children need can likely be found in the meals you put on your table, but the particulars may be quite different.
Biotracking will also help us stop acute and traumatic preventable deaths—by the millions. In 2018, a peer-reviewed study published by the team at InsideTracker and me, showed that biotracking and computer-generated food recommendations reduce blood sugar levels as efficiently as the leading diabetes drug, while optimizing other health biomarkers, too.
No source given, but it’s probably this study:
- Westerman, K., Reaver, A., Roy, C., Ploch, M., Sharoni, E., Nogal, B., … & Blander, G. (2018). Longitudinal analysis of biomarker data from a personalized nutrition platform in healthy subjects. Scientific reports, 8(1), 1-10.
The trend toward personalized approaches to health and medicine has resulted in a need to collect high-dimensional datasets on individuals from a wide variety of populations, in order to generate customized intervention strategies. However, it is not always clear whether insights derived from studies in patient populations or in controlled trial settings are transferable to individuals in the general population. To address this issue, a longitudinal analysis was conducted on blood biomarker data from 1032 generally healthy individuals who used an automated, web-based personalized nutrition and lifestyle platform. The study had two main aims: to analyze correlations between biomarkers for biological insights, and to characterize the effectiveness of the platform in improving biomarker levels. First, a biomarker correlation network was constructed to generate biological hypotheses that are relevant to researchers and, potentially, to users of personalized wellness tools. The correlation network revealed expected patterns, such as the established relationships between blood lipid levels, as well as novel insights, such as a connection between neutrophil and triglyceride concentrations that has been suggested as a relevant indicator of cardiovascular risk. Next, biomarker changes during platform use were assessed, showing a trend toward normalcy for most biomarkers in those participants whose values were out of the clinically normal range at baseline. Finally, associations were found between the selection of specific interventions and corresponding biomarker changes, suggesting directions for future study.
On results for BMI:
Changes in weight/body mass index are an important potential explanatory factor of the observed biomarker level changes over time. Due to weight data being collected independently of biomarker data, follow-up weights were available only for 428 participants and were not always concurrent with the follow-up blood tests, so were not analyzed formally with the blood biomarkers. However, for the participants with follow-up weights available, we observed a small decrease in mean BMI (mean change of −0.22, p = 0.043 from paired t-test).
Laughable.
![]()
Same paper, I don’t see anything specifically about blood sugars (as claimed in the book, I guess he refers to glucose), but this table has lots of p < .001’s. Seems like good evidence? Not really:
Next, we investigated the changes that occurred from baseline to follow-up in each biomarker separately. Results in our entire study population can be found in Supplementary Table S4. As these results are complicated by the difficulty of defining “improvement” in individuals with normal biomarker values at baseline, we focused on those individuals whose baseline values were outside of the clinically acceptable range for a given marker. It is more clear in these participants which direction of change constitutes improvement; further, these individuals would have received recommendations from the platform targeting that biomarker. A single “direction of risk” was defined for each biomarker as the direction in which individuals are most commonly found out-of-range (e.g. for blood glucose, an upper rather than lower limit was set). Participants tended to be out-of-range for only a few biomarkers each (full distribution in Fig. S1), with 906 individuals out-of-range for at least one biomarker. We analyzed the set of 17 biomarkers for which at least 20 participants were out-of-range at baseline, and observed substantial changes from baseline to follow-up in almost all markers examined (Table 2). As there was no randomization involved in this analysis, we do not infer any causality of platform use or identify which of its components may have driven the observed changes. Nonetheless, these observations suggest a potential influence of the platform on biomarkers in out-of-range individuals.
This is a typical regression towards the mean fallacy. If you sample people at time 1, then subset to those who were in the extreme (bad or good) part of the distribution at that time, then follow up to time 2, they will regress towards the mean no matter whatever you did to them. Authors discuss and reject this:
Second, the observed effect may reflect a statistical regression to the mean, which would occur due to our non-random selection of participants based on baseline values25. Because these baseline values are subject to random variation (e.g., due to technical variability in test results), the chosen subset of participants would be expected to show improved follow-up test values simply by chance. Though this may also explain some of the observed effect reported in Table 2, the numerous biomarker changes observed in the full population (Supplementary Table S4), many of whom had baseline values that were normal or at the opposite end of the spectrum, do not support this effect being the primary explanatory factor.
Their explanation does not make sense to me, and I didn’t look at their supplements. This is not a randomized trial anyway, so it cannot be too convincing even if they made regression towards the mean less plausible.
In general, the book is full of pompous references to studies in ‘good journals’:
Trading reproduction for repair, the sirtuins order our bodies to “buckle down” in times of stress and protect us against the major diseases of aging: diabetes and heart disease, Alzheimer’s disease and osteoporosis, even cancer. They mute the chronic, overactive inflammation that drives diseases such as atherosclerosis, metabolic disorders, ulcerative colitis, arthritis, and asthma. They prevent cell death and boost mitochondria, the power packs of the cell. They go to battle with muscle wasting, osteoporosis, and macular degeneration. In studies on mice, activating the sirtuins can improve DNA repair, boost memory, increase exercise endurance, and help the mice stay thin, regardless of what they eat. These are not wild guesses as to their power; scientists have established all of this in peer-reviewed studies published in journals such as Nature, Cell, and Science.
…
This wasn’t a case of the mainstream news media blowing things out of proportion. Highly respected scientific journals such as Science and Nature told pretty much the same story. It also wasn’t a case of scientists overstating their work. The truth is simply that, at the time, most researchers involved in the thirteen-year, $1 billion project agreed that we’d come as close as we possibly could—given the technology of the time—to identifying each of the 3 billion base pairs in our DNA.
…
In the aged SGS1 cells, the nucleolus looked as if it had exploded. Instead of a single red crescent swimming in a blue ocean, the nucleolus was scattered into half a dozen small islands. It was tragic and beautiful. The picture, which would later appear in the August 1997 issue of the prestigious journal Science, still hangs in my office.
…
When I began my career in this field, I dreamt of publishing just one study in a top-tier journal. During those years, our group was publishing one every few months.
And so on. This kind of thing may convince readers who don’t know better (unfortunately, that seems to be most doctors too), but this has the opposite effect on those who know better (those pesky 1%?). So let’s check out a bunch of the studies he cites as evidence. I am just going to search the book for “study” and “studies” and look them up for a quick check.
First one:
Yet even over the course of many thousands of years, their cells do not appear to have undergone any decline in function. Scientists call this “negligible senescence.” Indeed, when a team from the Institute of Forest Genetics went looking for signs of cellular aging—studying bristlecones from 23 to 4,713 years old—they came up empty-handed. Between young and old trees, their 2001 study found, there were no meaningful differences in the chemical transportation systems, in the rate of shoot growth, in the quality of the pollen they produced, in the size of their seeds, or in the way those seeds germinated.33
Refers to a book (M. D. LaPlante, Superlative: The Biology of Extremes), so I am going to skip.
But there’s a reason why the program was set to three kilometers. Mice simply don’t run that far. Yet these elderly mice were running ultramarathons.
Why? One of our key findings, in a study we published in 2018,42 was that when treated with an NAD-boosting molecule that activated the SIRT1 enzyme, the elderly mice’s endothelial cells, which line the blood vessels, were pushing their way into areas of the muscle that weren’t getting very much blood flow. New tiny blood vessels, capillaries, were formed, supplying badly needed oxygen, removing lactic acid and toxic metabolites from muscles, and reversing one of the most significant causes of frailty in mice and in humans. That was how these old mice suddenly became such mighty marathoners.
Study is here. This study has a bewildering number of statistical tests, all of which are shown only in large collections of figures. The sample sizes are small, and the p values aren’t generally reported, though many of them are very small judging by the asterisks. The small p values in such studies generally result from biological tests like whether some protein activated or not (easy). I can’t judge this one.
Next:
There are some simple tests to determine how biologically old you probably are. The number of push-ups you can do is a good indicator. If you are over 45 and can do more than twenty, you are doing well. The other test of age is the sitting-rising test (SRT). Sit on the floor, barefooted, with legs crossed. Lean forward quickly and see if you can get up in one move. A young person can. A middle-aged person typically needs to push off with one of their hands. An elderly person often needs to get onto one knee. A study of people 51 to 80 years found that 157 out of 159 people who passed away in 75 months had received less than perfect SRT scores.
No source given, but it’s this one. Check out fine, though not particularly doubtful to begin with.
Next:
Since the 1980s, a long-term study of calorie restriction in rhesus monkeys—our close genetic cousins—has produced stunningly compelling results. Before the study, the maximum known lifespan for any rhesus monkey was 40 years. But of twenty monkeys in the study that lived on calorie-restricted diets, six reached that age, which is roughly equivalent to their reaching 120 in human terms.
To hit that mark, the monkeys didn’t need to live on a calorie-restricted diet for their entire lives. Some of the test subjects were started on a 30 percent reduction regimen when they were middle-aged monkeys.10
CR works to extend the lifespan of mice, even when initiated at 19 months of age, the equivalent of a 60- to 65-year-old human, but the earlier the mice start on CR, the greater the lifespan extension.11 What these and other animal studies tell us is that it’s hard to “age out” of the longevity benefits of calorie restriction, but it’s probably better to start earlier than later, perhaps after age 40, when things really start to go downhill, molecularly speaking.
Studies are here and here. First study has:
Considering both males and females together, a statistically significant effect of CR in increasing survival was observed (Cox regression P=0.017; Supplementary Table 1). The hazard ratio (HR) of 1.865 (95% confidence interval (CI): 1.119–3.108) indicated that at any time-point the control monkeys had almost twice the rate of death when compared to CR animals.
…
Using multilevel modelling to investigate the relationship between adiposity and fasting glucose levels a significant relationship was identified for UW males only (P=0.005). A significant age by diet interaction was also detected (P=0.014), suggesting that the impact of age on the relationship between adiposity and glucoregulatory parameters is distinct for control and CR monkeys.
…
Cox proportional hazard regression modelling indicated that age-related conditions occurred at ∼2.7 times the rate in control animals compared to CR for UW monkeys (HR: 2.665; CI: 1.527–4.653; P=0.0006). In the NIA J/A cohort, age-related conditions occurred at twice the rate in control monkeys compared to CR (HR: 2.091; CI: 1.169–3.641; P=0.0125).
…
Similarly, for all NIA groups and for UW males, no relationship between adiposity and mortality was detected. For UW females, adiposity was associated with a modest reduction in risk for death (HR: 0.927; P=0.01) but only after correcting for age and diet. These data suggest that the influence of adiposity on survival risk is sexually dimorphic and changes with age. [no they don’t! run a proper interaction test!]
So their overall finding for CR is p = .017, and some of the supporting findings are more solid. Overall, it’s in the uh, maybe territory. Second study:
There was no difference in rapamycin levels between rapamycin-fed males and females although rapamycin-fed females consumed significantly more food per gram body mass than rapamycin-fed males (repeated measures analysis of variance p = .004).
…
Rapamycin feeding also significantly increased autophagy in white adipose tissue, with a greater increase observed in females (37%) compared with males (14%; p = .049, data not shown).
…
In contrast, rapamycin-fed females consumed less food per gram body mass than controls, and that difference increased with increasing age (p = .005, Supplementary Figure 4).
…
Temporal sleep patterns can be monitored in C57BL/6 mice by analyzing periods of inactivity of a critical length (37). Using this metric, sleep fragmentation, as assessed by number of sleep bouts per hour sleep, increased with age (Figure 3C, p = .028), as has been reported many times for both mice and humans. Overall, rapamycin feeding reduced sleep fragmentation (p = .006); however, the effect was strongly sex-specific. Upon analyzing the sexes separately, there was a nonsignificant trend for rapamycin-fed females to have more fragmented sleep than controls (p = .20), whereas rapamycin-fed males clearly had less fragmented sleep (p = .003). Rapamycin, in other words, maintained males—and only males—in a more youthful sleep pattern. This technique of high-throughput sleep assessment has only been validated in male C57BL/6 mice (33), so the implication of the female trend is unclear.
And so on, I got tired of looking at all their indirect measures. The key claim is about survival so, let’s look at that:
Survival. (A) Male control (n = 44) control and rapamycin-fed (n = 45), (B) Female control (n = 43) and rapamycin-fed (n = 45), and (C) Both sexes combined control (n = 87) and rapamycin fed (n = 90). Controls are shown in grey and rapamycin-fed mice in black. The survival curves were compared using the Cox-Mantel log-rank test, the p-values for males, females, and the sexes combined are shown; sex • treatment interaction was not significant (p = .532). A more powerful Cox Proportional Hazards Model found a significant effect of rapamycin feeding (p = .01) and sex (p = .02). See text for details.
So it’s in the doubtful territory. So far, not very convincing stuff. If I were a famous author, why would I pick so weak studies in my book? Imagine if IQ researchers picked so weak studies, this would be a field day for the commie activists to attack!
Next claim:
Today, human studies are confirming that once-in-a-while calorie restriction can have tremendous health results, even if the times of fasting are quite transient.
In one such study, participants ate a normal diet most of the time but turned to a significantly restricted diet consisting primarily of vegetable soup, energy bars, and supplements for five days each month. Over the course of just three months, those who maintained the “fasting mimicking” diet lost weight, reduced their body fat, and lowered their blood pressure, too. Perhaps most important, though, the participants had lower levels of a hormone made primarily in the liver called insulin-like growth factor 1, or IGF-1. Mutations in IGF-1 and the IGF-1 receptor gene are associated with lower rates of death and disease and found in abundance in females whose families tend to live past 100.15
Study is here. Actually, that’s just some cell study. The CR restriction study for humans is not cited. It’s hard to find based on just that description. But I’m pretty sure it’s this one, as the abstract is nearly word for word match.
Calorie restriction or changes in dietary composition can enhance healthy aging, but the inability of most subjects to adhere to chronic and extreme diets, as well as potentially adverse effects, limits their application. We randomized 100 generally healthy participants from the United States into two study arms and tested the effects of a fasting-mimicking diet (FMD)—low in calories, sugars, and protein but high in unsaturated fats—on markers/risk factors associated with aging and age-related diseases. We compared subjects who followed 3 months of an unrestricted diet to subjects who consumed the FMD for 5 consecutive days per month for 3 months. Three FMD cycles reduced body weight, trunk, and total body fat; lowered blood pressure; and decreased insulin-like growth factor 1 (IGF-1). No serious adverse effects were reported. After 3 months, control diet subjects were crossed over to the FMD program, resulting in a total of 71 subjects completing three FMD cycles. A post hoc analysis of subjects from both FMD arms showed that body mass index, blood pressure, fasting glucose, IGF-1, triglycerides, total and low-density lipoprotein cholesterol, and C-reactive protein were more beneficially affected in participants at risk for disease than in subjects who were not at risk. Thus, cycles of a 5-day FMD are safe, feasible, and effective in reducing markers/risk factors for aging and age-related diseases. Larger studies in patients with diagnosed diseases or selected on the basis of risk factors are warranted to confirm the effect of the FMD on disease prevention and treatment.
So let’s check the stats!
Next, we evaluated the effects of the FMD by assessing the changes in marker/risk factor values between baseline and 5 to 7 days after the end of the third cycle of the FMD and compared them to those occurring in the control arm within the same 3-month period (Fig. 2, Table 2, and table S2). Participants in the FMD arm (arm 2) lost on average 2.6 ± 2.5 kg (±SD) (P < 0.0001) of weight, which was due in part to a reduction in total body fat (absolute values and relative volume % of total mass) and trunk fat (absolute values) (Table 2 and table S2). Subjects on the control diet did not lose body weight (0.1 ± 2.1 kg). After controlling the false discovery rate (FDR) of 0.05 between the control and FMD groups, no change in the percentage of lean body mass was observed (relative to the total mass; P = 0.07), although absolute lean body mass was reduced in arm 2 (P = 0.004) (Table 2 and table S2). Waist circumference measured after three FMD cycles was reduced by 4.1 ± 5.2 cm (P = 0.0035 between groups). The FMD cycles also resulted in a decrease in IGF-1 concentrations of 21.7 ± 46.2 ng/ml (P = 0.0017 between groups). Systolic blood pressure was reduced by 4.5 ± 6.0 mmHg (P = 0.023 between groups), and diastolic blood pressure was reduced by 3.1 ± 4.7 mmHg (P = 0.053 between groups). Fasting glucose (P = 0.27), triglycerides (P = 0.27), cholesterol (total, P = 0.81; LDL, P = 0.50; HDL, P = 0.90), and the acute-phase inflammatory marker CRP (P = 0.27) did not differ significantly between groups. A graphical summary of these data is presented in Fig. 2. In conclusion, three cycles of the FMD reduced body weight, trunk and total body fat, blood pressure, and IGF-1 in comparison to a normal diet.
OK, some convincing p values. But the problem with this study are the atrocious drop-out rates:
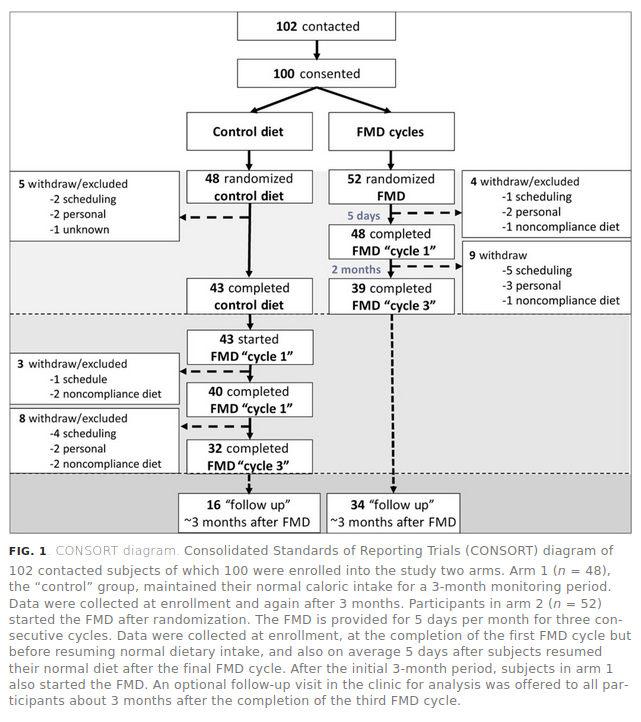
So, how would you fairly summarize this study? You enrolled 100 people, randomized them 48-52 to control vs. treatment. But then something like 67% of the control group dropped out and about 33% of the treatment group! What to make of such results? Not much.
OK, last claim:
One study of genetically engineered Ames dwarf mice, for instance, demonstrated that the function of brown fat is enhanced in these remarkably long-lived animals.43 Other studies have shown that animals with abundant brown fat or subjected to shivering cold for three hours a day have much more of the mitochondrial, UCP-boosting sirtuin, SIRT3, and experience significantly reduced rates of diabetes, obesity, and Alzheimer’s disease.44
Source given is just a news outlet summary (so maybe he didn’t read the study!), but it’s this study. It’s a mess because this study is actually in humans (n=5, cross-over) but the rat results are mentioned, but the sources they cited don’t appear to be the right study. Maybe you can find it, but my keyword search came up empty.
The big virus is coming for you
READY FOR THE WORST
In 1918—long before our modern, superfast, hyperconnected global transportation network took shape—a worldwide influenza pandemic that some historians believe originated in the United States killed more people in absolute numbers than any other disease outbreak in history.29 It was a violent death, with hemorrhage from mucous membranes, especially from the nose, stomach, eyes, ears, skin, and intestines.30 At a time in which the era of human flight was in its infancy and most people had never ridden in a car, the H1N1 virus found its way to some of the furthest reaches of our globe. It killed people on remote islands and in arctic villages. It killed without regard to race or national boundaries. It killed like a new Black Death. Average life expectancy in the United States plummeted from 55 to 40 years. It recovered, but not until more than 100 million people of all ages globally had had their lives cut short.This could happen again. And given how much more humans and animals are in contact and how much more interconnected our planet is now than it was a century ago, it could happen quite easily.
The gains in life expectancy we’ve witnessed over the past 120 years, and those to come, could be wiped out for a generation unless we address the greatest threat to our lives: other life-forms that seek to prey on us. It doesn’t matter if we live decades upon decades longer if a pandemic quickly snuffs out hundreds of millions of lives—negating and even rolling back the gains in average lifespan we will have achieved. Global warming is a long-term, critical issue to deal with, but one could also argue that, at least within our lifetimes, infections are our greatest threat.
Ensuring the next big outbreak never happens could be the greatest gift of the biotracking revolution. Individually, of course, real-time monitoring of vitals and body chemicals offers incredible benefits for optimizing health and preventing emergencies. Collectively, though, it could help us get ahead of a global pandemic.
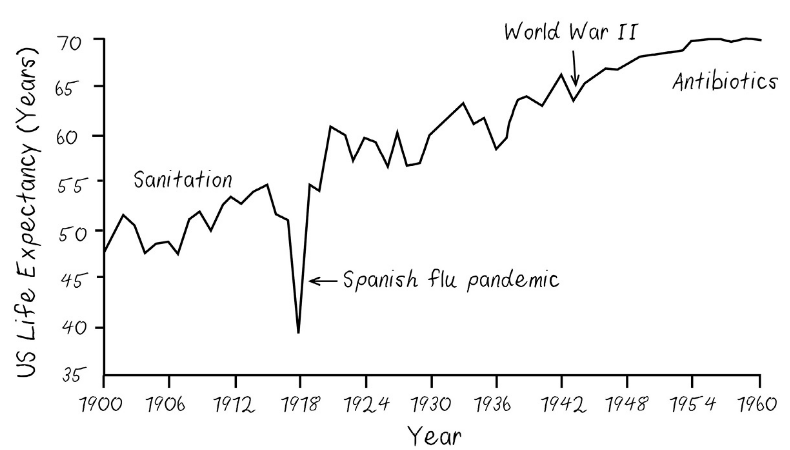
Eerily accurate. This book is from 2019. I guess Sinclair is now using COVID-19 as his go-to argument for more biotracking. I don’t disagree.
Lot of cringe woke stuff
Our oldest child, Alex, who at 16 hopes for a career in politics and social justice, has often struggled to see the future with the same optimism I do. Especially when you’re young, it is hard to see much of an arc to the moral universe, let alone one that bends toward justice.5
Alex grew up, after all, in a world that is quickly and disastrously warming; in a nation that has been at war for the better part of two decades; and in a city that suffered a terrorist attack on the people participating in one of its most cherished traditions, the Boston Marathon. And like so many other young people, Alex lives in a hyperconnected universe where news of one humanitarian crisis after the next, from Syria to South Sudan, is never far from the screen of a smartphone.
So I understand. Or I try to, at least. But it was disappointing to learn, one recent night, that Alex didn’t share the optimism I’ve always had about the future. Of course I’m proud that our kid has such a strong moral compass, but it was saddening to realize this more pessimistic view of the world casts a significant shadow over the way Alex sees my life’s work.
“Your generation, just like all the ones that came before, didn’t do anything about the destruction that is being done to this planet,” Alex told me that evening. “And now you want to help people live longer? So they can do even more damage to the world?”
I went to bed that night troubled. Not by our firstborn’s denouncement of me; of that, I admit, I was a little proud. We’ll never destroy the global patriarchy if our children don’t first practice on their fathers. No, what I was troubled by—what kept me up that night and has done so many since—were the questions that I simply could not answer.
Extremely cringe.
At the time of his death in 2003, Strom Thurmond was 100 years old and had served 48 years as a US senator. That Thurmond was a centenarian in Congress is no vice—we want our leaders to have experience and wisdom, as long as they aren’t stuck in the past. The travesty was that Thurmond somehow managed to keep his seat in spite of a long record of supporting segregation and opposing civil rights, including basic voting rights. At the age of 99, he voted to use military force in Iraq, opposed legislation to make pharmaceuticals more affordable, and helped kill a bill that would have added sexual orientation, gender, and disability to a list of categories covered by hate crimes legislation.29 After his death, the “family values” politician was revealed to have had a daughter with his family’s teenage African American housekeeper when he was 22, which was almost certainly an act of statutory rape under South Carolina law. Though he knew about the child, he never publicly acknowledged her.30 Thurmond lived in retirement only six months; those who were too young to vote then will have to live with the consequences of his votes for the rest of their lives.
We tend to tolerate a bit of bigotry among older people as a condition of the “age in which they grew up,” but perhaps also because we know we won’t have to live with it for long. Consider, though, a world in which people in their 60s will be voting not for another twenty or thirty years but for another sixty or seventy. Imagine a man like Thurmond serving in Congress not for half a century but for an entire century. Or, if it makes it easier to envision from your place on the political spectrum, picture the politician you despise more than any other holding power longer than any other leader in history. Now consider how long despots in far less democratic nations will cling to power—and what they will do with that power.
What will this mean for our world politically? If a steadfast driving force for kindness, tolerance, inclusivity, and justice suddenly ceases to exist, what will our world look like?
This is somewhat hilarious. I guess this can be considered a dark EA case for anti-aging, as this slows destructive communist youth movements.
If you were a member of the American upper middle class in the 1970s, you weren’t just enjoying a more affluent life, you had a longer one, too. Those in the top half of the economy were living an average of 1.2 more years than those in the bottom half.
By the early 2000s, the difference had increased dramatically. Those in the upper half of the income spectrum could expect nearly six additional years of life, and by 2018, the divide had widened, with the richest 10 percent of Americans living thirteen more years of life than the poorest 10 percent.40
The impact of this disparity cannot be overstated. Just by living longer, the rich are getting richer. And of course, by getting richer, they are living longer. Extra years offer more time to preside over family businesses, and more time for family investments to multiply exponentially.
Seems fishy, let’s check. Source is a NYT article from 2016. They don’t exactly link to the source, but I found a similar one by the same organization.
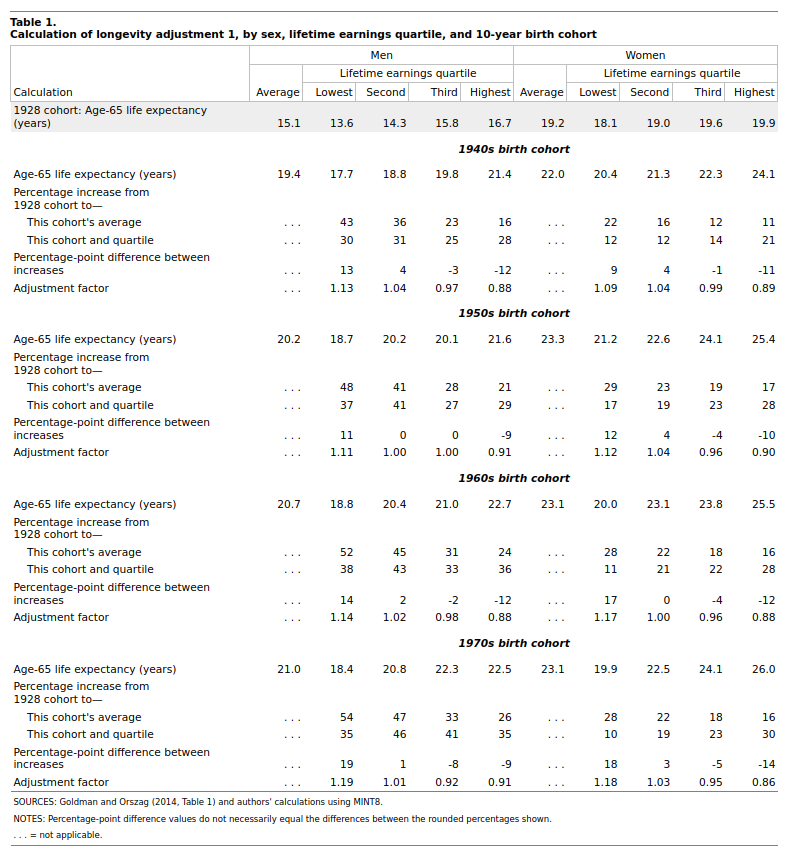
This one has just the 65+ remaining life expectancy by income groups, by birth cohorts. Let’s compare numerically:
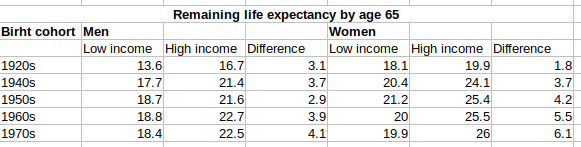
So the differences are indeed growing. This seems to be in line with Charles Murray’s Coming Apart stuff. I guess the big opioid killer hasn’t helped this pattern since.
We have already taken the first tenuous steps into a world that was predicted by the 1997 film Gattaca, a society in which technologies originally intended to assist in human reproduction are used to eliminate “prejudicial conditions,” but only for those who can afford them. In the coming decades, barring a safety issue or a global backlash against the unknown, we’ll likely see the increased ability and acceptance of gene editing globally, providing would-be parents with the option to limit disease susceptibility, choose physical traits, and even select intellectual and athletic abilities. Those of means who wish to give their children “the best possible start,” as a doctor tells two prospective parents in Gattaca, will be able to do so, and with longevity genes identified, they could be given the best possible finish, too. Whatever advantages genetically enhanced people will already have, they could be multiplied by virtue of economic access to longevity drugs, organ replacements, and therapies we haven’t even yet dreamed of.
Indeed, unless we act to ensure equality, we stand at the precipice of a world in which the über-rich could ensure that their children, and even their companion animals, live far longer than some poor people’s children do.
That would be a world in which the rich and poor will be separated not simply by differing economic experiences but by the very ways in which human life is defined—a world in which the rich will be permitted to evolve and the poor are left behind.
Yet . . .
Notwithstanding the potential that extending human longevity has to exacerbate some of the direst problems of our world—and indeed to give us new troubles in the decades to come—I remain optimistic about the potential of this revolution to change the world for the better.
We’ve been here before, after all.
More cringe. The elites will get any technology first since they finance the early development before it is marketable to the poorer. This drives down prices fast until most people can get it. This is the most effective way of doing things.
Heaney’s tragic poem echoes the sentiments expressed in a Life magazine article from 1959 titled “Old Age: Personal Crisis, U.S. Problem.”62
“The problem has never been so vast or the solution so inadequate,” the author wrote. “Since 1900, with better medical care, life expectancy has increased an average of 20 years. Today there are five times as many aged as in 1900 . . . the problem of old age comes almost overnight—when a man retires, after a woman’s husband dies.”
When I came upon the musty magazine in a Cape Cod bookstore on Old King’s Highway, I first marveled at how far gender equality has come since 1959, but then was struck by how little has changed in the way we fret about the calamity of the impending deluge of old people. Whatever will we do with them? Will they overwhelm our hospitals? What if they want to keep working?
The impact of this shift in the way many people view elders has been particularly hard felt in the workforce, where age discrimination is rampant. Hiring managers hardly bother to hide their prejudices. They view older workers as more likely to be sick, slow working, and incapable of handling new technologies.
Absolutely none of that is true, especially for people in management and leadership positions.
Yes, it used to be that technology was slow to catch on. But educated older people now use technology just as frequently as those under 65. Don’t forget, these are the generations who sent rockets to the moon, and invented the supersonic passenger jet and personal computer.
“Every aspect of job performance gets better as we age,” Peter Cappelli, the director of the Wharton Center for Human Resources, reported after he began to investigate the stereotypes that often surround older workers. “I thought the picture might be more mixed, but it isn’t. The juxtaposition between the superior performance of older workers and the discrimination against them in the workplace just really makes no sense.”63
Between 2012 and 2017, the average age of new CEOs at the largest companies in the United States increased from 45 to 50 years. Yes, it’s true that older people cannot work physically the same way they did when they were 20, but when it comes to management and leadership, it’s the opposite. Consider some examples of leadership: Tim Cook, Apple’s CEO, is currently 58; Bill Gates, Microsoft cofounder, is 63; Indra Noori, who recently stepped down as CEO of PepsiCo and now sits on Amazon’s board, is 63; and Warren Buffett, the CEO of the investment firm Berkshire Hathaway, is 87. These people are not what you’d call technophobes.
It’s bad enough when companies allow themselves to be deprived of great workers because of untrue stereotypes. But this is done at a national and international scale, sidelining millions of people in the best years of their work lives—all because of old ideas about age that aren’t true now and that are going to be even less true in the near future. Thanks to the Age Discrimination in Employment Act of 1967, individuals in the United States over age 40 are legally protected from employment discrimination based on age. But in Europe, most workers are forced to retire in their mid-60s, including professors, who are just getting good at what they do. The best ones move to the United States so they can keep on innovating.
It’s Europe’s loss, and it’s completely backward.
…
Right now, about half of the people in the United States and Europe between the ages of 50 and 74 are suffering from a mobility impairment. About a third have hypertension. More than one in ten is fighting heart disease or diabetes. More than one in twenty is suffering from cancer or lung disease.65 Many are fighting several of these diseases at once. Even so, they far outperform the young at most mental tasks, writing and vocabulary, and leadership.
Bizarre! Has he never googled age decline of intelligence? It looks like this:
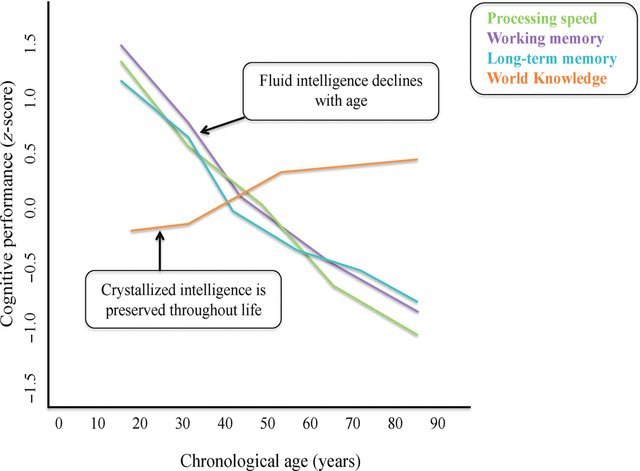
Sinclair must be living in a parallel world. Try explaining computer stuff to anyone above 50, watch as they type looking at the keyboard with one finger at a time, and it’s endless torture. Creativity decreases with age as well, this is obvious stuff.
And the final prediction for Metaculus (question here, pending review/approval):
Labs like mine, focused specifically on developing innovative therapies to treat, stop, and reverse aging, will no longer be rare. There will be one or more at every health science university in the world.
And there should be, because there is no shortage of scientists lining up to enlist in this army. Right now, I and other researchers who study aging are being besieged by eager, experienced, and absolutely brilliant youths who want nothing more than to devote their lives to the fight to stop aging. For lab heads like me, it’s a virtual buyer’s market. There are far more people who want to work in aging than there are labs they can work in. What this means is that there are a lot of people who, despite being wicked smart and raring to tackle the aging problem, are having to work in other fields or other professions. This will soon change.
The first nations to define aging as a disease, both in custom and on paper, will change the course of the future. The first places to provide large amounts of public funding to augment the fast-growing private investments in this field will prosper in kind. It will be their citizens who benefit first. Doctors will feel comfortable prescribing medicines, such as metformin, to their patients before they become irreversibly frail. Jobs will be created. Scientists and drug makers will flock to that country. Industries will thrive. Their national budget will see a significant return on investment. Their leaders’ names will be in the history books.
And the holders of the patents, the universities and the companies, will have more money than they know what to do with.
I bet not. The first parts of extending aging will be somatic, so we will mainly end up with more old people who cannot do much meaningful work due to cognitive decline, so they will be an increasingly large net drain the economy, not a gain. The best way forward for anti-aging should in fact focus on the most costly part of it, which is mental decline, something that ironically Sinclair seems in denial about!
This was an enjoyable read, but my checking of various claims in the book showed they were often grossly misleading or statistically dodgy, or even untraceable to sources. I didn’t change my opinion about trusting claims in medical or health books, on the contrary, I am now even more skeptical. I look forward to the day someone writes an actual trustable book on this topic. How difficult can it be to avoid studies with p = .04, >50% drop-out rates and so on? Seems like 101 critical thining.