Since the 1930s, people are getting older and older before having children, as the importance of careers and education to people seems to increase without stop. As older people are less fecund, we now have a lot more issues with infertility. The medical establishment’s solution to this is to work on methods for increasing fertility of older people, that is, IVF. This works by extracting eggs, saving them for later (freezing), and eventually choosing an optimal embryo for implantation (embryo selection). There are also some fertility drugs that may work to improve egg release (ovulation). One might say in other words that the medical establishment is trying to do as well as it can given the political reality that one cannot tell people about how careerism is bad for their family plans. Instead, company offer to pay their female employees to freeze their eggs in the hope that they can use them later.
All the above methods are really for dealing with older would be mothers, not would be fathers. As men produce semen all their lives, there is no shortage of them. However, there is a quality problem, as the semen is reduced in quality with age. The way to avoid this problem would be to freeze down sperm. I’ve previously been asked about whether I thought this would be worth the money, and I said no, and I haven’t done it myself either. I said no for the usual reasons about skepticism of older findings not replicating, and because the theoretical expectations are quite small. As mentioned, men produce semen all life long (not before puberty I think). This is done in the gonads (the testicles) by cells that themselves divide, or derive from cells that divide (spermatogenesis). This means that these cells will accumulate mutations during the aging of the father, and these will then also be found in the semen. Here the most obvious idea would be to sequence a bunch of sperm and check the mutation rates, and whether these mutations seem to be the usual random point mutations, or if they rather more often involve larger mutations, which would have larger negative effects. Still, it must be remembered that most mutations, including large ones, would be expected to have zero effect on the phenotype, as the genome is mainly full of old viruses and otherwise inactive DNA (long repeats and the like).
Maternal age would also have some effects, but not in the same way. Females are born with all their eggs, and these don’t divide during their lifetime, but rather sit around in a kind pre-egg stasis until activated. Activated here means that before ovulation happens, some pre-egg cells are given some hormonal treatment in the ovaries and then are finalized for a functional egg (oogenesis). Sometimes more than one egg is made ready and this can then result in dizygotic twins (and triplets etc.). So, unlike the cell division that accumulates mutations for men, eggs would not accumulate such errors. They can still become worse with age, and of course, mutations can happen due to cosmic rays hitting the DNA. Michael Woodley has been writing about the theoretical effects of paternal age on intelligence in various works like this one, but the empirical results have been more lackluster. Arslan et al 2017 is notable for using a very large dataset to study the effect of parental age of later offspring fitness (fertility):
- Arslan, R. C., Willführ, K. P., Frans, E. M., Verweij, K. J., Bürkner, P. C., Myrskylä, M., … & Penke, L. (2017). Older fathers’ children have lower evolutionary fitness across four centuries and in four populations. Proceedings of the Royal Society B: Biological Sciences, 284(1862), 20171562.
Higher paternal age at offspring conception increases de novo genetic mutations. Based on evolutionary genetic theory we predicted older fathers’ children, all else equal, would be less likely to survive and reproduce, i.e. have lower fitness. In sibling control studies, we find support for negative paternal age effects on offspring survival and reproductive success across four large populations with an aggregate N > 1.4 million. Three populations were pre-industrial (1670–1850) Western populations and showed negative paternal age effects on infant survival and offspring reproductive success. In twentieth-century Sweden, we found minuscule paternal age effects on survival, but found negative effects on reproductive success. Effects survived tests for key competing explanations, including maternal age and parental loss, but effects varied widely over different plausible model specifications and some competing explanations such as diminishing paternal investment and epigenetic mutations could not be tested. We can use our findings to aid in predicting the effect increasingly older parents in today’s society will have on their children’s survival and reproductive success. To the extent that we succeeded in isolating a mutation-driven effect of paternal age, our results can be understood to show that de novo mutations reduce offspring fitness across populations and time periods.
Their findings are basically that evolution still seems to work a little bit. Children born of older fathers die off a bit more, and are less successful in their own marriages. This would be consistent with evolution selecting against the new mutations. If this was strong enough, it could keep these mutations from accumulating over time in the genome gene pool. This is an important matter because if mutations keep accumulating, the collective gene pool of humans will slowly deteriorate, theoretically, to the point of near extinction where civilization would collapse and natural selection would kick back in to kill off the high mutational load persons. Dark stuff! It is for this reason that it is inevitable to do some kind of eugenics. The damage to the gene pool is always accumulating in the absence of strong selection against it.
Other researchers have tried to compute on theoretical grounds how much these new mutations should be able to cause higher rates of mental illness:
- Gratten, J., Wray, N. R., Peyrot, W. J., McGrath, J. J., Visscher, P. M., & Goddard, M. E. (2016). Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nature genetics, 48(7), 718-724.
The offspring of older fathers have higher risk of psychiatric disorders such as schizophrenia and autism. Paternal-age-related de novo mutations are widely assumed to be the underlying causal mechanism, and, although such mutations must logically make some contribution, there are alternative explanations (for example, elevated liability to psychiatric illness may delay fatherhood). We used population genetic models based on empirical observations of key parameters (for example, mutation rate, prevalence, and heritability) to assess the genetic relationship between paternal age and risk of psychiatric illness. These models suggest that age-related mutations are unlikely to explain much of the increased risk of psychiatric disorders in children of older fathers. Conversely, a model incorporating a weak correlation between age at first child and liability to psychiatric illness matched epidemiological observations. Our results suggest that genetic risk factors shared by older fathers and their offspring are a credible alternative explanation to de novo mutations for risk to children of older fathers.
In terms of IQ, there’s an Israeli study that looked at this already back in 2005:
- Malaspina, D., Reichenberg, A., Weiser, M., Fennig, S., Davidson, M., Harlap, S., … & Knobler, H. Y. (2005). Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatric genetics, 15(2), 117-125.
Background A robust association between advancing paternal age and schizophrenia risk is reported, and genetic changes in the germ cells of older men are presumed to underlie the effect. If that is so, then the pathway may include effects on cognition, as those with premorbid schizophrenia are reported to have lower intelligence. There are also substantial genetic influences on intelligence, so de novo genetic events in male germ cells, which accompany advancing paternal age, may plausibly influence offspring intelligence.
Objective An association of paternal age with IQ in healthy adolescents may illuminate the mechanisms that link it to schizophrenia.
Method We examined the association of paternal age and IQ scores using the Israeli Army Board data on 44 175 individuals from a richly described birth cohort, along with maternal age and other potential modifiers.Results A significant inverted U-shaped relationship was observed between paternal age and IQ scores, which was independent from a similar association of IQ scores with maternal age. These relationships were not significantly attenuated by controlling for multiple possible confounding factors, including the other parent’s age, parental education, social class, sex and birth order, birth weight and birth complications. Overall, parental age accounted for ∼2% of the total variance in IQ scores, with later paternal age lowering non-verbal IQ scores more than verbal IQ scores.
Conclusion We found independent effects of maternal and paternal age on offspring IQ scores. The paternal age effect may be explained by de novo mutations or abnormal methylation of paternally imprinted genes, whereas maternal age may affect fetal neurodevelopment through age-related alterations in the in-utero environment. The influence of late paternal age to modify non-verbal IQ may be related to the pathways that increase the risk for schizophrenia in the offspring of older fathers.
Their plots look like this. Unadjusted results:
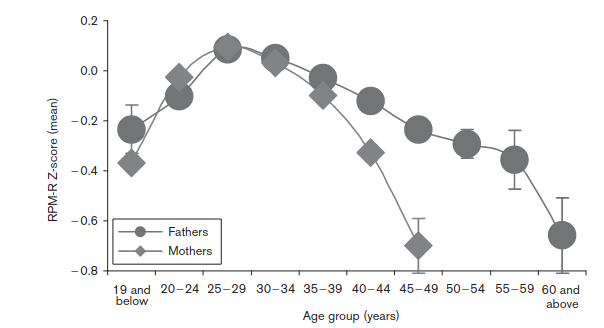
So, without making any adjustments, the optimal breeding age seems to be late 20s. Earlier or later means lower IQs. This is not consistent with mutations, because younger people have fewer mutations. These must be self-selection biases. So they adjusted for this, insofar as possible, and the results looked like this:
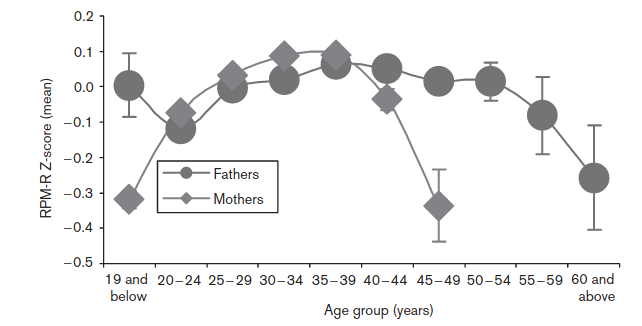
They adjusted for: “presence of asphyxia, sex, parental socio-economic status, parent’s education, birth weight and parity”. We don’t actually want to adjust for birth weight, as this is obviously to some extent genetically caused and thus can be caused by paternal age caused mutations itself, so it’s an over-adjustment. In any case, we see the same shape for women, which cannot be due to mutations, and the male pattern is non-monotonic, also inconsistent with mutations causing the damage. So we can’t really say how much these results reflect self-selection bias versus a true effect of paternal age mutations causing lower IQs.
So that brings us to the new study, from Taiwan:
- Wang, S. H., Wu, C. S., Hsu, L. Y., Lin, M. C., Chen, P. C., Thompson, W. K., & Fan, C. C. (2022). Paternal age and 13 psychiatric disorders in the offspring: a population-based cohort study of 7 million children in Taiwan. Molecular Psychiatry, 1-
Although paternal age has been linked to certain psychiatric disorders, the nature of any causal relationship remains elusive. Here, we aimed to comprehensively assess the magnitude of a wide range of offspring’s psychiatric risk conferred by paternal age, leveraging a pedigree inferred from covered-insurance relationship (accuracy >98%) in Taiwan’s single-payer compulsory insurance program. We also examined whether there is an independent role of paternal age and explored the potential effect of parental age difference. A total cohort of 7,264,788 individuals born between 1980 and 2018 were included; 5,572,232 with sibling(s) were selected for sibling-comparison analyses and 1,368,942 and 1,044,420 children with information of paternal-grandparents and maternal-grandparents, respectively, were selected for multi-generation analyses. Using inpatient/outpatient claims data (1997–2018), we identified schizophrenia, autism, bipolar disorder (BPD), attention deficit-hyperactivity disorder (ADHD), major depressive disorder (MDD), eating disorder (ED), substance use disorder (SUD), mental retardation (MR), tic disorder, obsessive-compulsive disorder (OCD), anxiety, and somatoform disorder. We identified suicides using death certificates. Logistic regression analysis was used to estimate the paternal/maternal/grand-paternal age association with psychiatric risk in the offspring. The total cohort and sibling-comparison cohort resulted in similar estimates. Paternal age had a U-shaped relationship with offspring’s MDD, ED, SUD, and anxiety. A very young maternal age (<20 years) was associated with markedly higher risk in offspring’s SUD, MR, and suicide. Older paternal age (>25 years) was linearly associated with offspring’s schizophrenia, autism, BPD, ADHD, MDD, ED, SUD, MR, OCD, anxiety, and suicide. Older grand-paternal age was linearly associated with offspring’s schizophrenia, autism, ADHD, and MR. Dissimilar parental age was positively associated with offspring’s ADHD, MDD, SUD, MR, anxiety, and suicide, and negatively associated with offspring’s OCD. This comprehensive assessment provides solid evidence for the independent role of paternal age in psychiatric risk in the offspring and clarifies the significance of both early parenthood and delayed paternity.
The study as described is nearly perfect: they have a huge dataset, high quality demographics data, they have diagnoses of a wide variety of disorders, and they have sibling comparisons. They don’t have intelligence per se, but they have mental retardation, the left tail (which is not entirely optimal as it can also be caused by major genetic defects rather than a change in the mean intelligence). The sibling comparison data means that we are comparing children of the same two biological parents, but who had children at different times. We can then control not only for the birth orders (first vs. second born etc), but the ages of the parents at the time. This removes any bias from self-selection because, recall, these are the same parents. Parental age effects seen within sibling pairs with a control for birth order are hard to attribute to anything else but effects of age itself. I mean, one could ascribe them to environmental effects of parental aging on child-rearing (or indirect genetic ones), but that’s a stretch. These psychiatric traits usually have high heritabilities and small to null shared environment effects, and it’s not likely that parental skills would markedly deteriorate between say age 30 and 50 in a way that could increase, say, the schizophrenia rate by 100%. But that’s what their plots show. First, the unadjusted results between families:
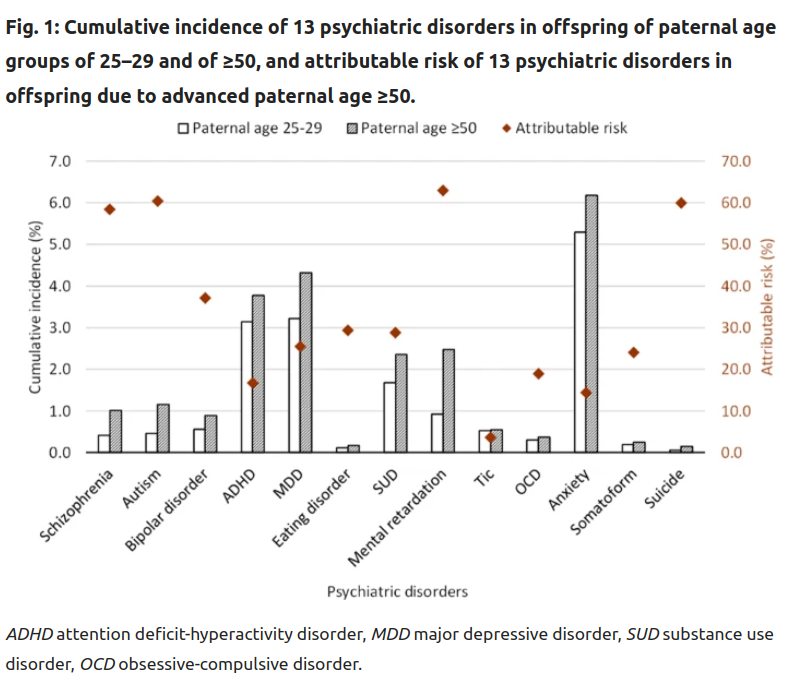
So here we are looking at lifetime risks of getting one of these diagnoses, based on their fathers’ age: there’s an age 25-50 group and a 50+ group. The latter shows elevated rates for every diagnosis, some of them quite large, >100% (e.g. schizophrenia), some of them near-0% (e.g. tic disorder). In my mind, the varying effect sizes are not very plausible given that these diagnosis to a large degree share their genetic causes (P factor), and because they have similarly strongly polygenic genetic architectures. The latter means that there are a lot of causal variants for the disorders, which means that they are roughly equally likely to get hit by some new mutation. Think about a hypothetical disorder mainly caused by variation in one gene. It would be very unlikely to be hit by any mutation, whereas a trait that’s determined by genes distributed all over the genome would have a much higher chance to get hit. This is called the mutational target. Larger target, more effects of mutations.
Anyway, these results have the same biases as usual, in that it is not entirely random when different men breed in their lives, so we know the results are biased. If more mentally ill people breed later, this can explain this pattern. That’s the self-selection model that everybody is thinking of, and which was the basis of the theoretical study cited above. But the results for siblings look much the same!
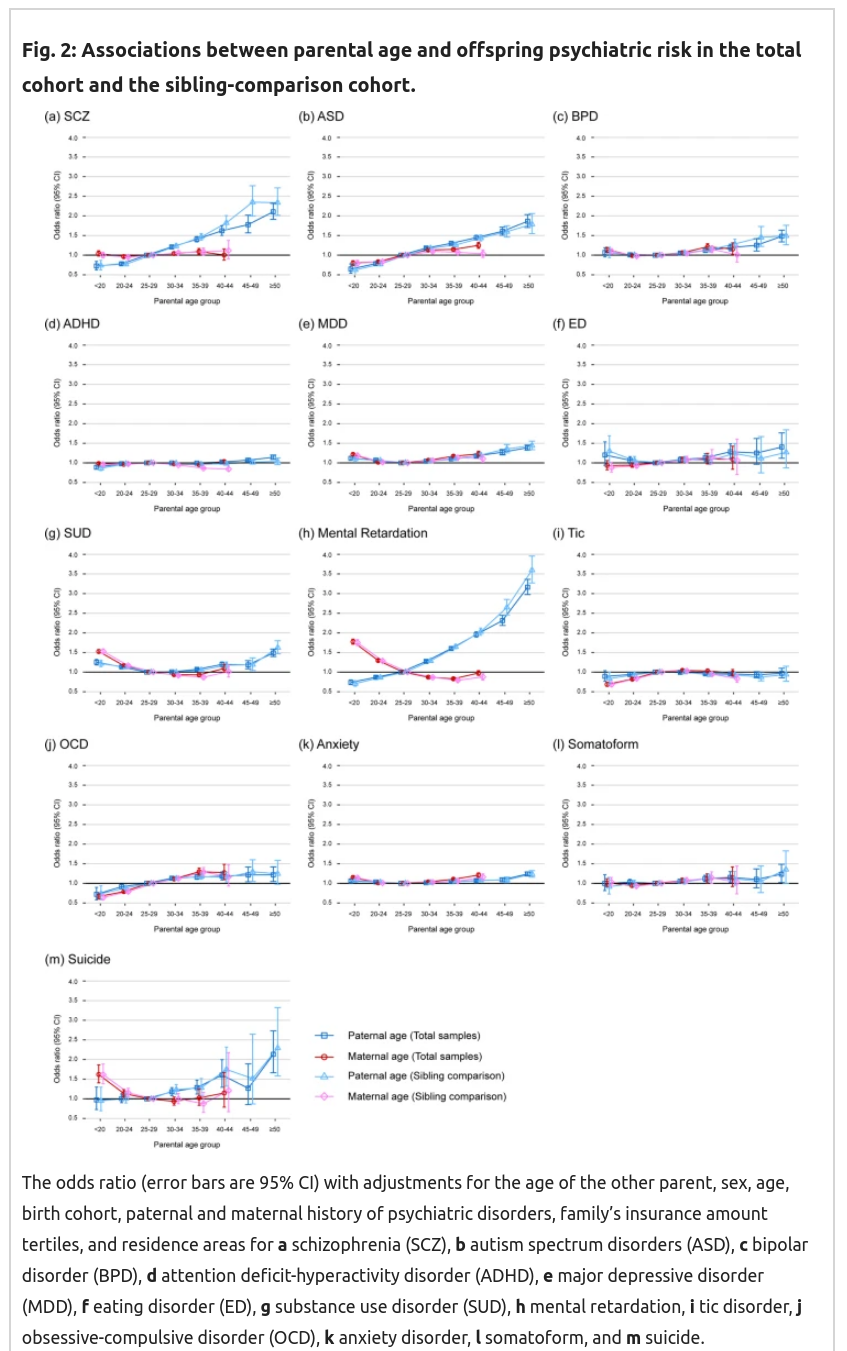
Recall here that based on genetic theory, mutation effects must be monotonically increasing with age, I don’t know if they must be strictly linear, but it should be close. We are particularly interested in the comparisons between the squares and the triangles, as these represent the difference between between-family effects (with controls!) and the sibling comparison. The latter is immune to self-selection bias. So these estimates should be different if the previous results were due to these biases to some extent. But as you can see above, they find that they are not much different, if at all. That’s extremely surprising, as this implies there’s essentially no self-selection bias. That seems impossible. Rather strangely, the female results are also mostly null, and some show some seemingly impossible positive effects (for suicide, retardation, drug abuse) that cannot be due to genetic effects, unless one is now claiming that women’s eggs haven’t properly matured before age 25ish. The reverse female results for retardation are particularly unbelievable as of course chromosomal abnormalities (aneuploidies) are a large part of the reason why older women get more retarded children (i.e. Down syndrome and many other syndromes). It is not likely that most of these women received embryo selection with screening for aneuploidies as these data go back to the 1980s.
My overall conclusion from these data: they look too good to be true. There’s implausible reverse effects, lack of expected self-selection effects, mysterious variation between disorders (no effect on ADHD but large effect on the genetically highly correlated ASD, .59 in this review). It doesn’t make any sense. I’m not buying it.
That is, not completely. Obviously seeing a seemingly well done study like this with a massive sample size indicating the opposite of what I thought makes me reconsider. So maybe men should be freezing their sperm on the probability that I’m wrong about this study, and the effects really are large like that.
Edited to add: Swedish study from 2014 shows the same thing, ish
It seems my worries were not sensible. One big Iranian reader points me to this Swedish study:
- D’Onofrio, B. M., Rickert, M. E., Frans, E., Kuja-Halkola, R., Almqvist, C., Sjölander, A., … & Lichtenstein, P. (2014). Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA psychiatry, 71(4), 432-438.
Objective
Examine the associations between advancing paternal age at childbearing and numerous indices of offspring morbidity.
Setting
Population-based cohort study in Sweden.
Participants
All individuals born in Sweden 1973–2001 (N=2,615,081), with subsets of the data used to predict childhood/adolescent morbidity.
Design
We estimated the risk for psychiatric and academic morbidity associated with advancing paternal age using several quasi-experimental designs, including the comparison of differentially exposed siblings, cousins, and first-born cousins.
Exposure
Paternal age at childbearing
Main outcomes
Psychiatric (autism, ADHD, psychosis, bipolar disorder, suicide attempt, and substance use problem) and academic (failing grades and low educational attainment) morbidity.
Results
In the population, advancing paternal age was associated with increased risk for some psychiatric disorders (e.g. autism, psychosis, and bipolar disorders) but decreased risk for the other indices of morbidity. In contrast, the sibling-comparison analyses indicated that advancing paternal age had a dose-response relationship with every index of morbidity, with the magnitude of the associations being as large or larger than the estimates in the entire population. Compared to offspring born to fathers 20–25 years old, offspring of fathers 45 years+ were at heightened risk for autism (Hazard Ratio [HR]=3.45, 95% Confidence Intervals [CI]=1.62–7.33), ADHD (HR=13.13, CI=6.85–25.16), psychosis (HR=2.07, CI=1.35–3.20), bipolar disorder (HR=24.70, CI=12.12–50.31), suicide attempts (HR=2.72, CI=2.08–3.56), substance use problems (HR=2.44, CI=1.98–2.99), failing a grade (Odds Ratio [OR]=1.59, CI=1.37–1.85), and low educational attainment (OR=1.70, CI=1.50–1.93) in within-sibling comparisons. Additional analyses using several quasi-experimental designs obtained commensurate results, further strengthening the internal and external validity of the findings.
Conclusions and Relevance
Advancing paternal age is associated with increased risk for psychiatric and academic morbidity, with the magnitude of the risks being as large or larger than previous estimates. These findings are consistent with the hypothesis that new genetic mutations occurring during spermatogenesis are causally related to offspring morbidity.
Their results:
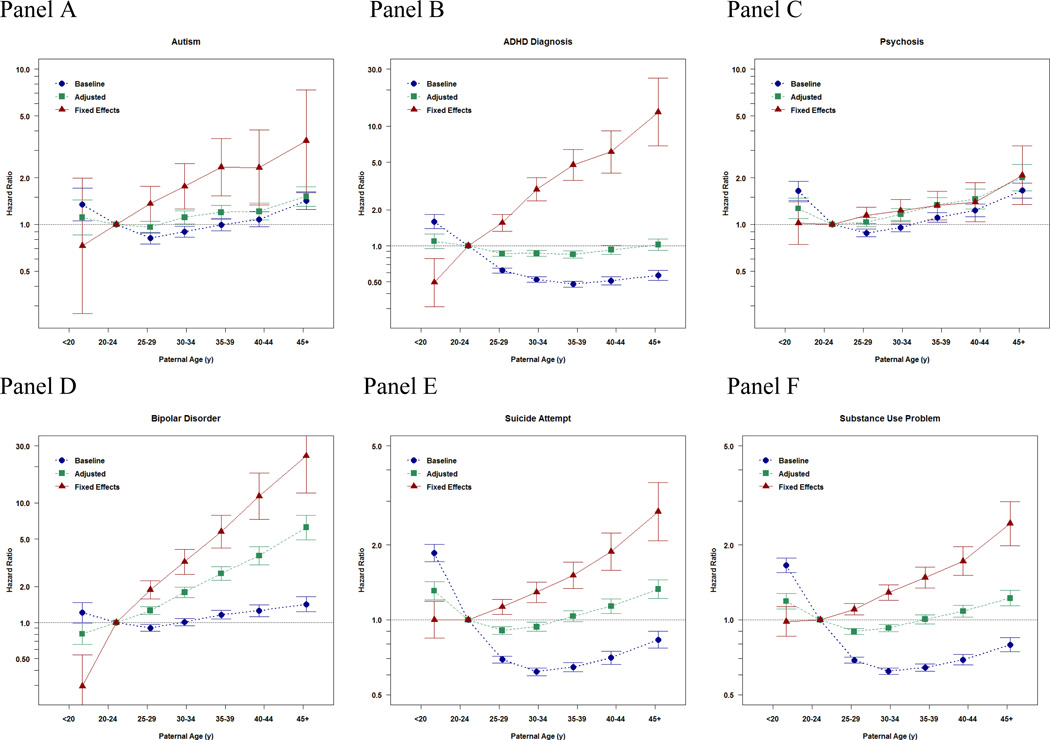
Notice the consistency of the findings across different diagnoses, as one would expect per a genetic cause. They also looked at school outcomes:

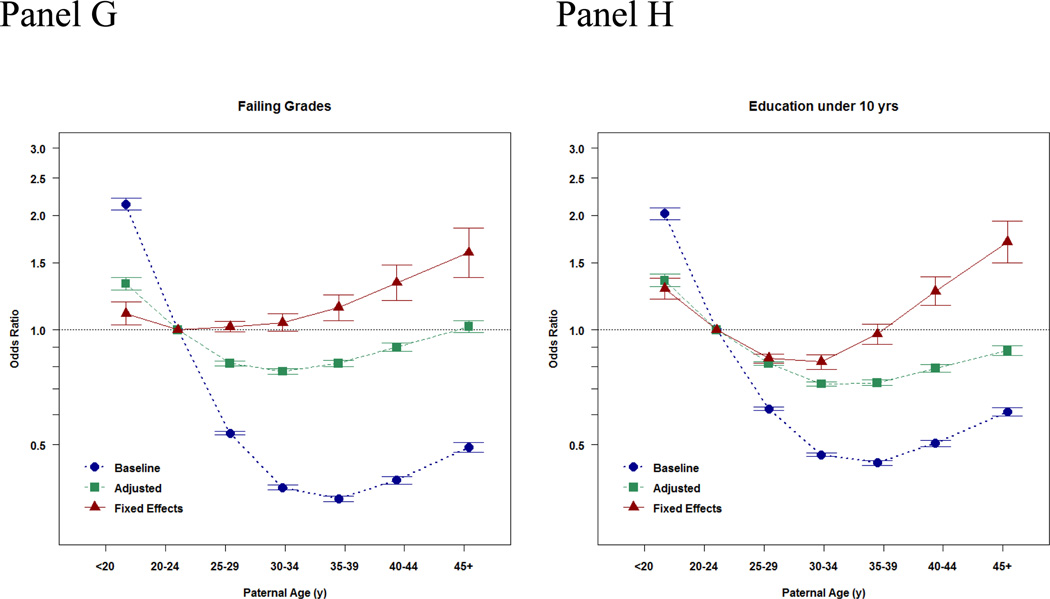
These show a non-monotonic pattern, which is very odd! Indeed, bizarre, and rather impossible if this is caused by paternal age mutational load. Their appendix has results for IQ as well as school grades as a continuous outcome:
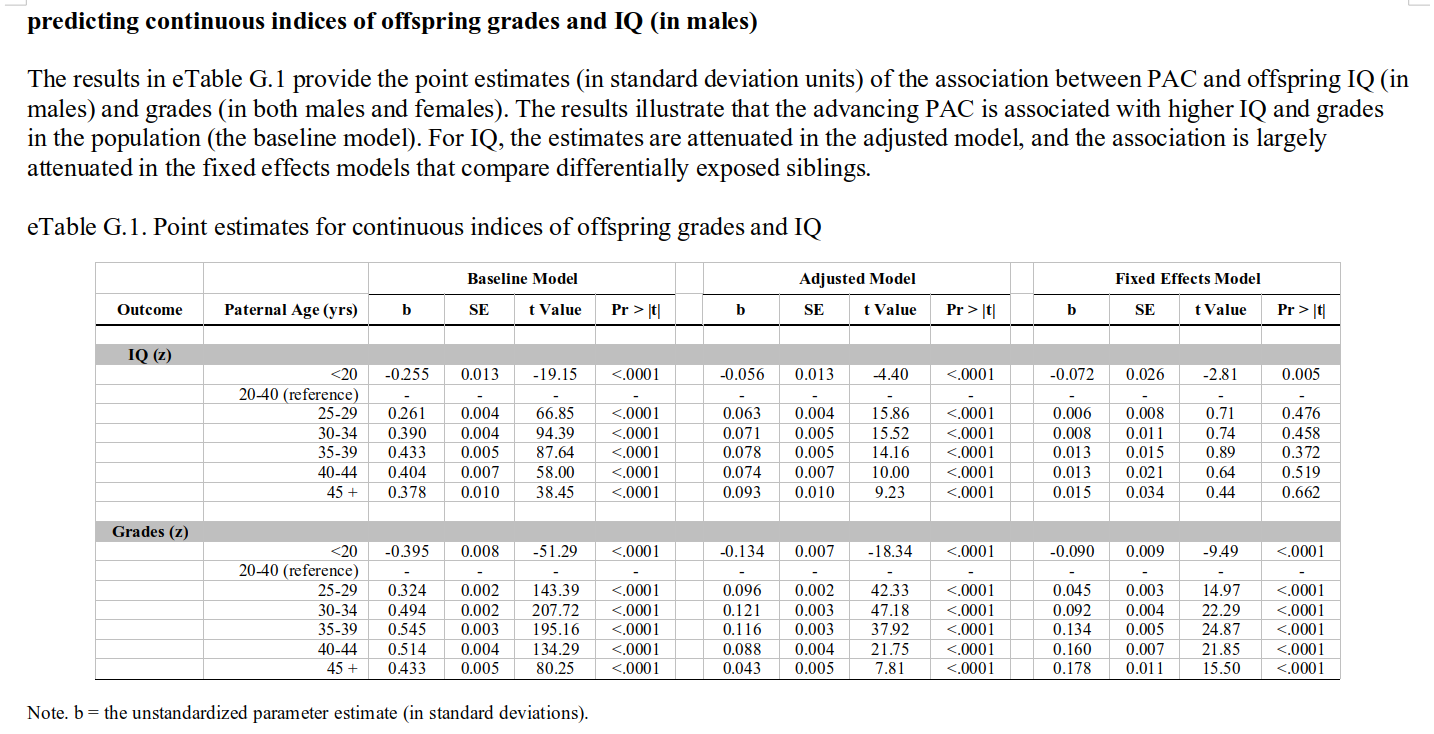
Here we see the expected positive association in the baseline model, and after adjustment (since these are imperfect). In the sibling models, there is still a positive association for grades, while the IQ slope is also there, but not more than one would expect by noise (I think it would be p < .05 if one fit the right model, but they opted for a bunch of dummies in their model). That is to say: these associations are in the opposite direction of what would expect based on paternal age mutational effects. These should result in lower IQs, not higher IQs and higher grades! Indeed, the findings are rather mysterious, as the plot above shows that the chance of having failing grades goes up with paternal age, but so does the mean grade… which means that the variation must be increasing too. Increasing variation is actually a prediction from a paternal age genetic model, but an increasing mean is the opposite of the prediction.
These findings are very mysterious. Maybe there is some issue with diagnostic bias, where kids with older parents/fathers are more often sent to a psychiatrist for behavioral issues, and end up with more diagnosis, despite not being more mentally ill than those of younger parents. The use of diagnoses allows for this kind of bias, and it’s hard to rule out. One good idea would be to use the Swedish army interview data for non-cognitive ability. This is an interview given to every male in the older cohorts, so there cannot be any diagnostic bias, and the interviewers don’t know the ages of the draftees. This kind of data has previously been used to show the advantage of first borns for non-cognitive ability. Check out this awesome study.