Because on this blog we love anything that has to do with calipers.
The most obscure weapon in the HBD arsenal? Goes like this:
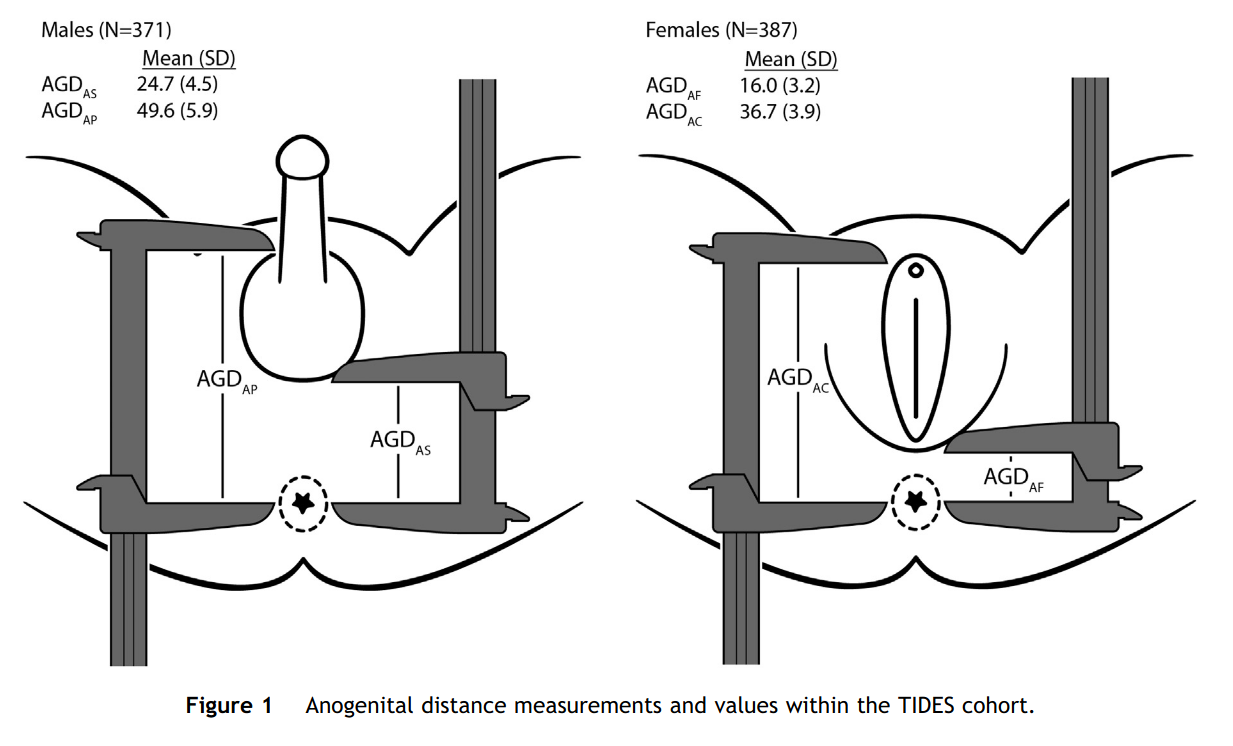
Figure from this 2015 study.
Alright, so we see that males have a lot bigger distance than women, so we figure this has something to do with sex differentiation and thus hormones. So maybe it is a useful proxy for testosterone, either prenatally or adult or something else cool? Let’s look over the first studies Google Scholar gives us:
-
Mira‐Escolano, M. P., Mendiola, J., Mínguez‐Alarcón, L., Melgarejo, M., Cutillas‐Tolín, A., Roca, M., … & Torres‐Cantero, A. M. (2014). Longer anogenital distance is associated with higher testosterone levels in women: a cross‐sectional study. BJOG: An International Journal of Obstetrics & Gynaecology, 121(11), 1359-1364.
Objective
Animal models have suggested that anogenital distance (AGD) at birth reflects androgen levels during in utero development and predicts adult AGD. A recent study showed an association between perineal length and androgen levels in men, suggesting that serum testosterone levels in adulthood will depend on factors involved during the fetal period. The aim of this study is to assess the relationship between AGD measures and reproductive hormone levels in women.
Design
Cross-sectional study conducted between February and November 2011.
Setting
University-affiliated fertility clinics.Population100 young college students.Methods
Physical and gynaecological examinations were conducted on university students. All participants provided a blood sample for determination of reproductive hormones and completed an epidemiological questionnaire on lifestyles and gynaecological history. We used multiple linear regression analysis to examine the associations between perineal length measurements [anus-fourchette (AGDAF) and anus-clitoris (AGDAC)] and reproductive hormone levels.
Main outcome measures
Anogenital distance measurements and reproductive hormone levels.
Results
In the multiple linear regression analyses, AGDAF was positively associated with serum testosterone levels. Serum testosterone increased 0.06 ng/ml (95%CI 0.01, 0.10; P = 0.02) for each 1-cm increase in AGDAF. None of the measurements was associated with other reproductive hormones.
Conclusions
Anogenital distance may predict normal reproductive development in women, and may be a new tool of potential clinical interest to evaluate ovarian function. Our results suggest that serum testosterone levels in adulthood may depend on factors operating in the prenatal period.
Abstract gives us a p = .02.

So a bunch of nothing, a p= .03 and a .02. One unadjusted p = .002 is the only thing that is found here. They had 2 measures of AGD and 5 hormones and 2+ variants since they were inconsistent in what to adjust for across models. But they tried at least 20 models here.
-
Hotchkiss, A. K., Lambright, C. S., Ostby, J. S., Parks-Saldutti, L., Vandenbergh, J. G., & Gray Jr, L. E. (2007). Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicological Sciences, 96(2), 335-345.
A rat study. I am going to shrug as it was too complicated to understand (read: a series of small studies).
-
Barrett, E. S., Hoeger, K. M., Sathyanarayana, S., Abbott, D. H., Redmon, J. B., Nguyen, R. H., & Swan, S. H. (2018). Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. Journal of developmental origins of health and disease, 9(3), 307-314.
Polycystic ovary syndrome (PCOS) affects approximately 7% of reproductive age women. Although its etiology is unknown, in animals, excess prenatal testosterone (T) exposure induces PCOS-like phenotypes. While measuring fetal T in humans is infeasible, demonstrating in utero androgen exposure using a reliable newborn biomarker, anogenital distance (AGD), would provide evidence for a fetal origin of PCOS and potentially identify girls at risk. Using data from a pregnancy cohort (The Infant Development and the Environment Study), we tested the novel hypothesis that infant girls born to women with PCOS have longer AGD, suggesting higher fetal T exposure, than girls born to women without PCOS. During pregnancy, women reported whether they ever had a PCOS diagnosis. After birth, infant girls underwent two AGD measurements: anofourchette distance [AGD-AF] and anoclitoral distance [AGD-AC]. We fit adjusted linear regression models to examine the association between maternal PCOS and girls’ AGD. In total, 300 mother-daughter dyads had complete data and 23 mothers reported PCOS. AGD was longer in the daughters of women with a PCOS diagnosis compared to daughters of women with no diagnosis (AGD-AF:β=1.21, p=0.05; AGD-AC:β=1.05, p=0.18). Results were stronger in analyses limited to term births (AGD-AF:β=1.65, p=0.02; AGD-AC:β=1.43, p=0.09). Our study is the first to examine AGD in offspring of women with PCOS. Our results are consistent with findings that women with PCOS have longer AGD and suggest that during PCOS pregnancies, daughters may experience elevated T exposure. Identifying the underlying causes of PCOS may facilitate early identification and intervention for those at risk.
Everything in the abstract is dodgy. Their table isn’t much better:

Lot of dodgy p values.
-
Eisenberg, M. L., Jensen, T. K., Walters, R. C., Skakkebaek, N. E., & Lipshultz, L. I. (2012). The relationship between anogenital distance and reproductive hormone levels in adult men. The Journal of urology, 187(2), 594-598.
Purpose:
Anogenital distance is a marker for endocrine disruption in animal studies in which decreased distance has been associated with testicular dysfunction. In this study we investigated whether anogenital distance was associated with reproductive hormone levels in adult men.
Materials and Methods:
A total of 116 men (mean age 36.1 ± 8.0 years) were evaluated at an andrology clinic in Houston. Anogenital distance (the distance from the posterior aspect of the scrotum to the anal verge) and penile length were measured using digital calipers. Testis size was estimated by physical examination. Linear regression was used to determine correlations between genital measurements and hormone levels.
Results:
Anogenital distance (r = 0.20, p = 0.03) and penile length (r = 0.20, p = 0.03) were significantly associated with serum testosterone levels while total testis size was not (r = 0.17, p = 0.07). No relationship between genital length and luteinizing hormone, follicle-stimulating hormone or estradiol was identified. After adjusting for age the serum testosterone increased by 20.1 ng/dl (95% CI 1.8, 38.4; p = 0.03) for each 1 cm increase in anogenital distance. On multivariable models no statistically significant relationship existed between penile length and testosterone levels. Moreover men with hypogonadal testosterone levels (less than 300 ng/dl) had a significantly shorter anogenital distance compared to men with higher testosterone levels (31.6 vs 37.3 mm, p = 0.02).
Conclusions:
Anogenital distance may provide a novel metric to assess testicular function in men. Assuming that anogenital distance at birth predicts adult anogenital distance, our findings suggest a fetal origin for adult testicular function.
Are these people even trying? Actually, they are trying too hard. Everything is even in the abstract.
-
Manno III, F. A. M. (2008). Measurement of the digit lengths and the anogenital distance in mice. Physiology & behavior, 93(1-2), 364-368.
-
Do, R. P., Stahlhut, R. W., Ponzi, D., Vom Saal, F. S., & Taylor, J. A. (2012). Non-monotonic dose effects of in utero exposure to di (2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reproductive toxicology, 34(4), 614-621.
Another 2 rodent studies. I am going to disregard.
-
Kareem, A. J., Owa, J. A., & Elusiyan, J. B. E. (2020). Estimations of total serum testosterone levels in Nigerian term neonates at birth using anogenital distance measurements. Journal of Pediatric Endocrinology and Metabolism, 33(5), 631-638.
estosterone is required but is expensive and technically difficult to assay. Therefore, the measurement of anogeni-tal distance, which is non-invasive and cheap, could be used to estimate total serum testosterone in neonates. The objective if this study is to determine the relationship between total serum testosterone and anogenital distance and estimate total serum testosterone levels in term neo-nates using measurements of anogenital distance.Methods: This was a prospective cross-sectional study. Consecutive healthy term neonates were recruited in the first 72 h of postnatal life. Anogenital distance was meas-ured with a digital vernier calliper. Total serum testoster-one was determined using enzyme linked immunoassay.Results: A total of 240 term neonates comprising 124 (51.7%) males and 116 (48.3%) females were studied. The overall mean anogenital distance was 19.7 (7.7) mm and 26.5 (3.7) mm for males which was more than twice 12.4 (2.3) mm for females (t = 35.3, p < 0.001, 95% confidence inter-val [CI], 13–14). The overall mean total serum testosterone level was 267.1 (204.8) ng/dL; and 357.4 (241.7) ng/dL in males which was more than twice of 170.6 (80.7) ng/dL for females (t = 7.9, p < 0.001, 95% CI, 144–221). There was positive correlation between total serum testosterone and anogenital distance (r = 0.425, p < 0.001). The correlation was stronger in males than in females. The linear regres-sion equation was as follows: total serum testosterone (ng/dL) = 44.3 + 11.3*AGD (mm) with 95% CI, 8–14.Conclusions: The known value of anogenital distance could be used to estimate total serum testosterone levels in term neonates.
Study not on Scihub, the p values are good, correlation too good, I am going to go out on a limb and say this is a fraudulent study. Request the raw data and check.
-
Barrett, E. S., Parlett, L. E., Sathyanarayana, S., Liu, F., Redmon, J. B., Wang, C., & Swan, S. H. (2013). Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiology & behavior, 114, 14-20.
In animal models, prenatal stress programs reproductive development in the resulting offspring, however little is known about effects in humans. Anogenital distance (AGD) is a commonly used, sexually dimorphic biomarker of prenatal androgen exposure in many species. In rodents, prenatally stressed males have shorter AGD than controls (suggesting lower prenatal androgen exposure), whereas prenatally stressed females have longer AGD than controls (suggesting greater prenatal androgen exposure). Our objective was to investigate the relationship between stressful life events in pregnancy and infant AGD. In a prospective cohort study, pregnant women and their partners reported exposure to stressful life events during pregnancy. Pregnancies in which the couple reported 4+ life events were considered highly stressed. After birth (average 16.5 months), trained examiners measured AGD in the infants (137 males, 136 females). After adjusting for age, body size and other covariates, females born to couples reporting high stress had significantly longer (i.e. more masculine) AGD than females born to couples reporting low stress (p=0.015). Among males, high stress was weakly, but not significantly, associated with shorter AGD. Our results suggest prenatal stress may masculinize some aspects of female reproductive development in humans. More sensitive measures of prenatal stress and additional measures of reproductive development are needed to better understand these relationships and clarify mechanisms.
So abstract gives us a subgroup with p = .015 after a just of method fishing. Shrug.
Rest of the results are as you would expect: nothing.
-
McCoy, S. J., & Shirley, B. A. (1992). Effects of prenatal administration of testosterone and cortisone on the reproductive system of the female rat. Life sciences, 50(9), 621-628.
-
Morova, M., SENKO, T., Olexova, L., Dzirbikova, Z., & KRŠKOVÁ, L. (2020). A mixture of diethylhexyl, diisononyl and dibutyl phthalate decreased anogenital distance, postnatal testosterone levels, and changed social behavior in Wistar rats. Physiological research, 69.
More rat studies.
-
Toprak, T., & Tokat, E. (2019). Does anogenital distance change with age?. Andrologia, 51(11), e13431.
It was known that in animals, anogenital distance (AGD), an indicator of prenatal androgen environment, was a stabile phenotype that persists throughout life. However, it is not known whether this applies to humans. In this study, we aimed to investigate whether anogenital distance is stable or not in males. We evaluated a total of 130 men targeted for group 1 (fathers) and group 2 (sons) in each 65 participants. AGD, the distance from anus to the posterior base of the scrotum, was measured with digital calipers. Anthropometric characteristics and testosterone levels of groups were recorded. We studied anogenital index (AGI), by dividing AGD by BMI to control bias of the weight and height, which could influence the measurement of AGD. The mean age of fathers was 61.5 ± 10.2 and that of children was 32.1 ± 5.48 (p = .00). The mean AGD scores were 55.46 ± 10.36 vs. 60.21 ± 10.04 (p = .09) and the mean total testosterone levels were 3.6 ± 1.47 vs. 5.45 ± 2.3 (p = .00) in group 1 and 2 respectively. There was no significant difference in height and weight between the two groups. AGD decreases with age, but further longitudinal studies are needed.
Looks like they were hoping for something more, once they didn’t find anything, came up with this totally not HARKing framing. The only thing they show in abstract is that dads are older than sons (p = .00, impressive), and sons are higher in in T level. Anyway, so if we check their paper, we see they left out their non-finding for T level and AGD.
-
Jain, V. G., Goyal, V., Chowdhary, V., Swarup, N., Singh, R. J., Singal, A., & Shekhawat, P. (2018). Anogenital distance is determined during early gestation in humans. Human Reproduction, 33(9), 1619-1627.
STUDY DESIGN, SIZE, DURATION
A prospective descriptive cohort study was performed using data from randomly selected neonates (n = 205) born at a single center over a period of 1 year (August 2015 to August 2016).
PARTICIPANTS/MATERIALS, SETTING, METHODS
AGDs in male (n = 117) and female infants (n = 88) together with penile width, glans girth and stretched penile length were measured by trained caregivers. Gestation ranged from 22 to 41 weeks and infants were examined within 24 h of birth (within 48–72 h in very sick preterm infants after clinical stabilization). AGD-1 was measured from the center of the anus to the posterior base of scrotum in males or to the posterior fourchette in females. AGD-2 was measured from the center of the anus to the anterior base of the penis in males or to the clitoris in females. Sex steroid hormones (testosterone, 17-OH progesterone (17-OHP) and androstenedione) were measured in serum prepared from umbilical cord blood samples taken at birth, using liquid chromatography-tandem mass spectrometry.
MAIN RESULTS AND THE ROLE OF CHANCE
Males had a significantly lower gestational age (mean ± SD; 34.6 ± 4.9 versus 36.1 ± 4.1 weeks, P = 0.04), and a significantly longer AGD-1 (mean ± SD; 21.6 ± 6.0 versus 12.7 ± 3.8 mm, P < 0.001) and AGD-2 (41.9 ± 8.7 versus 33.9 ± 7.1 mm, P = 0.004) compared to female infants, respectively. The cord serum testosterone levels were significantly higher for male than female infants [median, interquartile range; 13.0 (7.3, 20.5) versus 4.1 (2.5, 5.9), ng/dl, P < 0.001]. There was no difference in levels of 17-OHP (P = 0.697) or androstenedione (P = 0.601) between the two sexes. On multiple regression analysis after adjusting for potential confounders, none of the AGD’s in both males and females correlated with any sex steroid hormonal levels. We also provide normative charts for penile length, penile width and glans girth in preterm and term infants.
So they found nothing useful for AGD as a proxy for anything useful other than just being male or female. Sample size about 200 infants.
-
Parra, M. D., Mendiola, J., Jørgensen, N., Swan, S. H., & Torres‐Cantero, A. M. (2016). Anogenital distance and reproductive parameters in young men. Andrologia, 48(1), 3-10.
The purpose of this study was to assess the relationship between anogenital distance (AGD) measures and semen quality and serum reproductive hormone levels in Caucasian young men from southern Spain. Two variants of AGD [from the anus to the posterior base of the scrotum (AGDAS) and to the cephalad insertion of the penis (AGDAP)] were assessed in 215 university students. Semen parameters (semen volume, sperm concentration, total sperm counts, motility and morphology) and serum reproductive hormones (follicle stimulating hormone, luteinising hormone, inhibin B, testosterone, calculated free testosterone, oestradiol and sex hormone-binding globulin) were also determined. Associations between AGD measures and the semen quality and reproductive hormone levels were tested using multiple regression analyses. Overall, median sperm concentration was 44.0 × 106 ml−1 (5th–95th percentiles: 8.9–129 × 106 ml−1), median total sperm count was 121 × 106 (18.0–400 × 106), and mean (SD) testosterone level was 21.7 nmol/l (6.9). Mean (SD) AGDAS and AGDAP measures were 48.3 mm (11.6) and 128 mm (12.0) respectively. In the multivariable analysis, AGD measures were not associated with any semen parameters or any of the reproductive hormone levels, which is in contrast to results of studies of US young men or infertile men. Further research is warranted to confirm these findings.
They find the same as above. Sample size about 200 male students. It contracts the other study of semen analysis above but the sample size is twice as large. Bad omen.
-
Toprak, T., Şahin, A., Akgul, K., Kutluhan, M. A., Ramazanoglu, M. A., Yilmaz, M., … & Verit, A. (2020). The relationship between anogenital distance and lifelong premature ejaculation. Andrology, 8(2), 353-357.
Objectives
The aim of this study was to investigate the association between lifelong premature ejaculation and anogenital distance.
Materials and methodsThe study included 140 participants: 70 with lifelong premature ejaculation (group 1) and 70 without any ejaculatory complaints (group 2). Premature Ejaculation Diagnostic Tool and stopwatch intravaginal ejaculatory latency time were recorded from all participants in order to evaluate ejaculatory function. Two variants of anogenital distance were measured: anogenital distance (from anus to the posterior base of the scrotum) from anus to the posterior base of the scrotum and anogenital distance (from anus to the cephalad insertion of the penis) to the cephalad insertion of the penis. We compared differences between groups and correlations between anogenital distance variants and patients’ characteristics.
ResultsThe groups were similar in terms of age, BMI, and total testosterone levels. The mean anogenital distance (from anus to the posterior base of the scrotum) scores were 59.45 ± 10.76 vs. 55.02 ± 10.13 (p = 0.01), and anogenital distance (from anus to the cephalad insertion of the penis) scores were 128.37 ± 22.2 vs. 126.78 ± 16.21 (p = 0.63) in groups 1 and 2, respectively. Significant correlation was observed between anogenital distance (from anus to the posterior base of the scrotum) and Premature Ejaculation Diagnostic Tool scores (r = 0.199, p = 0.019) and intravaginal ejaculatory latency time (r = −0.185, p = 0.028). There were no statistically significant differences between anogenital distance (from anus to the posterior base of the scrotum) scores and total testosterone levels and between anogenital distance (from anus to the cephalad insertion of the penis) and Premature Ejaculation Diagnostic Tool scores or intravaginal ejaculatory latency time.
ConclusionsThese results suggest that longer anogenital distance is associated with higher possibility of lifelong premature ejaculation. However, further studies are needed to confirm our results.
Authors nicely inform us by their p values their study is shit.
-
Eisenberg, M. L., Hsieh, M. H., Walters, R. C., Krasnow, R., & Lipshultz, L. I. (2011). The relationship between anogenital distance, fatherhood, and fertility in adult men. PloS one, 6(5), e18973.
Methods
A cross sectional study of consecutive men being evaluated for infertility and men with proven fertility was recruited from an andrology clinic. Anogenital distance (the distance from the posterior aspect of the scrotum to the anal verge) and penile length (PL) were measured using digital calipers. ANOVA and linear regression were used to determine correlations between AGD, fatherhood status, and semen analysis parameters (sperm density, motility, and total motile sperm count).
Findings
A total of 117 infertile men (mean age: 35.3±17.4) and 56 fertile men (mean age: 44.8±9.7) were recruited. The infertile men possessed significantly shorter mean AGD and PL compared to the fertile controls (AGD: 31.8 vs 44.6 mm, PL: 107.1 vs 119.5 mm, p<0.01). The difference in AGD persisted even after accounting for ethnic and anthropomorphic differences. In addition to fatherhood, on both unadjusted and adjusted linear regression, AGD was significantly correlated with sperm density and total motile sperm count. After adjusting for demographic and reproductive variables, for each 1 cm increase in a man’s AGD, the sperm density increases by 4.3 million sperm per mL (95% CI 0.53, 8.09, p = 0.03) and the total motile sperm count increases by 6.0 million sperm (95% CI 1.34, 10.58, p = 0.01). On adjusted analyses, no correlation was seen between penile length and semen parameters.
Conclusion
A longer anogenital distance is associated with fatherhood and may predict normal male reproductive potential. Thus, AGD may provide a novel metric to assess reproductive potential in men.
Same people as before, same dataset too, same dubious p values. OK, maybe infertile men have a smaller distance. I can imagine this as some infertility is caused by birth defects and odd intersex stuff, which may also change AGD but does not mean AGD works as a proxy for anything interesting for normal people.
-
Glintborg, D., Jensen, R. C., Schmedes, A. V., Brandslund, I., Kyhl, H. B., Jensen, T. K., & Andersen, M. S. (2019). Anogenital distance in children born of mothers with polycystic ovary syndrome: the Odense Child Cohort. Human Reproduction, 34(10), 2061-2070.
STUDY QUESTION
Are higher testosterone levels during pregnancy in women with polycystic ovary syndrome (PCOS) associated with longer offspring anogenital distance (AGD)?
SUMMARY ANSWER
AGD was similar in 3-month-old children born of mothers with PCOS compared to controls.
WHAT IS KNOWN ALREADY
AGD is considered a marker of prenatal androgenization.
STUDY DESIGN, SIZE, DURATION
Maternal testosterone levels were measured by mass spectrometry at Gestational Week 28 in 1127 women. Maternal diagnosis of PCOS before pregnancy was defined according to Rotterdam criteria. Offspring measures included AGD from anus to posterior fourchette (AGDaf) and clitoris (AGDac) in girls and to scrotum (AGDas) and penis (AGDap) and penile width in boys and body composition (weight and BMI SD scores) at age 3 months.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The study was part of the prospective study, Odense Child Cohort (OCC), and included mothers with PCOS (n = 139) and controls (n = 1422). The control population included women with regular menstrual cycles (<35 days) before conception and no signs of androgen excess (hirsutism and/or acne).
MAIN RESULTS AND THE ROLE OF CHANCE
AGD measures were comparable in offspring of women with PCOS compared to controls (all P > 0.2) despite significantly higher maternal levels of total testosterone (mean: 2.4 versus 2.0 nmol/l) and free testosterone (mean: 0.005 versus 0.004 nmol/l) in women with PCOS versus controls (both P < 0.001). In women with PCOS, maternal testosterone was an independent positive predictor of offspring AGDas and AGDap in boys. Maternal testosterone levels did not predict AGD in girls born of mothers with PCOS or in boys or girls born of women in the control group.
LIMITATIONS, REASONS FOR CAUTION
The diagnosis of PCOS was based on retrospective information and questionnaires during pregnancy. Women participating in OCC were more ethnically homogenous, leaner, more educated and less likely to smoke compared to the background population. Our study findings, therefore, need to be reproduced in prospective study cohorts with PCOS, in more obese study populations and in women of other ethnicities.
Ah, some Danes! Some good stuff? Idea is that we look at cases of PCOS who are elevated in maternal T levels. By obvious implication then, everything else equal, their offspring should have different AGD means. They report a subgroup finding, only works for boys? Let’s look closer.
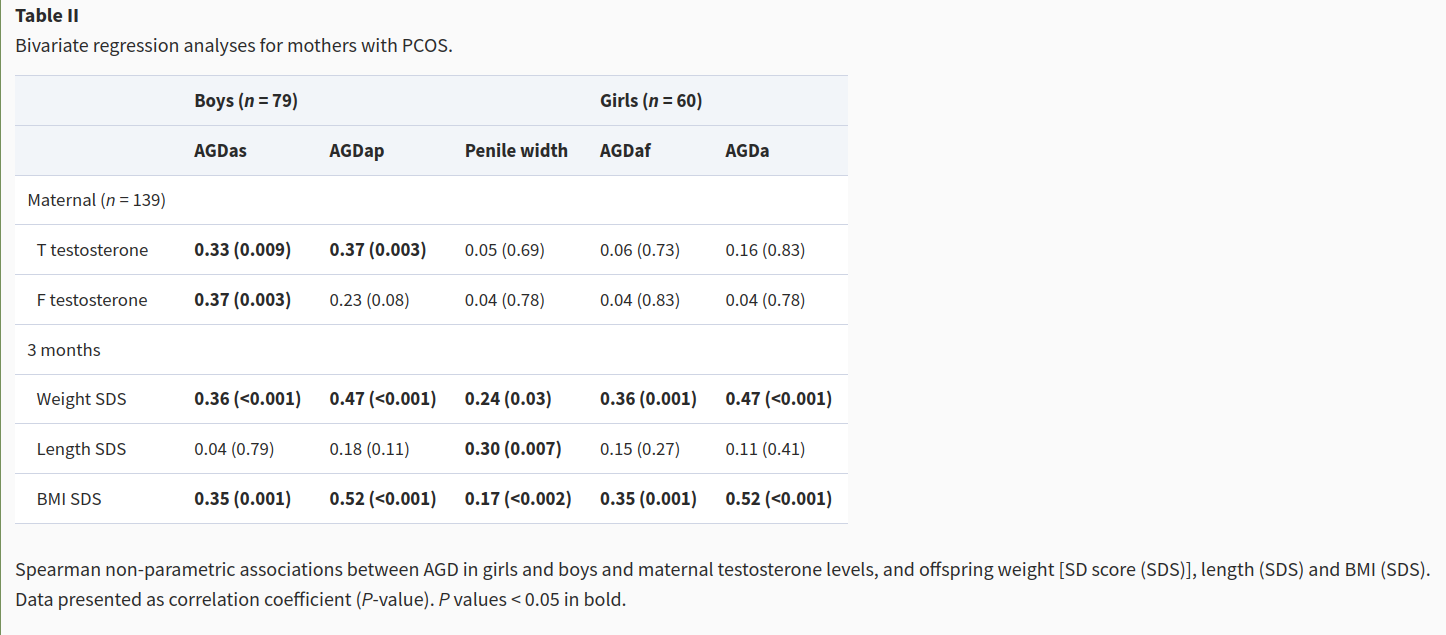
OK, so there’s a bunch of not dubious stuff, but all of these are actually just related to size, so really just showing us that larger babies have larger AGD. Duh. This small subgroup sample shows correlations for boys, p values not so dubious .009 and .003. Hmm!

Their table 3 is only showing p-hacked stuff. Table 4 even worse, and they only left out one of their models in Table 3 (AGDac).
So in the best case, this study shows that maternal T predicts offspring AGD compared to other women (“during the 27th–28th week of gestation”). So that’s not really a proxy for own T level. Unless it correlates a lot between mother’s gestation in that week and self later on. Maybe? Well, hold on. They are hiding their main finding in a brief paragraph:
Measures of AGD were not significantly associated with maternal testosterone levels in girls and boys aged 3 months (Supplementary Table SII).
What? So they have been doing this tiny subgroup analysis at length, while their big sample showed NO relationship??

Checking their suppplement reveals the answer to that question is: yes.
I stop here. I covered all relevant studies on page 1-2. With the exception of the probably fraudulent African study (prior is very high), everything else was either negative or obviously p-hacked to death. A reasonable observer will apply the Bayesian modus tollens here. Although authors might have all gotten really lucky and all, and these are just OK findings with p values in the magic publication zone, we already know of all the cheating, and the most likely way to get such results across people and studies is there is a collective delusion and p-hacking fest. Lessons to be learned from the candidate gene era remain unlearned by large areas of science.

