Research funding for COVID is amazing. A lot of academics got and continue to get grants promising to do research on COVID. Unfortunately, COVID kinda died out recently and the public largely stopped caring in many countries, so what to do with the grant money? Well, there’s long COVID! This term already has like 4 alternative names listed on Wikipedia — post-COVID-19 syndrome, post-COVID-19 condition, post-acute sequelae of COVID-19 (PASC), and chronic COVID syndrome (CCS) — so maybe it can be milked a lot going forward. Worse, maybe the medias attention to this will cause a lot of mentally ill people to identify as being long-COVIDers going forward. This kind of copycat behavior for the mentally ill is well known in psychiatry. We see it already for the whole transsexual craze, so why not add another?
Well, that is one very contrarian view. It assumes that long COVID is substantially not real in some sense. I mean, it is real in the sense that people report long COVID symptoms, and there’s an entire industry producing papers about how people who report long COVID are different from those who don’t. There are several meta-analyses that you can easily find on Google Scholar. Let’s take one example:
- Chen, C., Haupert, S. R., Zimmermann, L., Shi, X., Fritsche, L. G., & Mukherjee, B. (2022). Global Prevalence of Post COVID-19 Condition or Long COVID: A Meta-Analysis and Systematic Review. The Journal of Infectious Diseases.
Background
This study aims to examine the worldwide prevalence of post-coronavirus disease 2019 (COVID-19) condition, through a systematic review and meta-analysis.Methods
PubMed, Embase, and iSearch were searched on July 5, 2021 with verification extending to March 13, 2022. Using a random-effects framework with DerSimonian-Laird estimator, we meta-analyzed post-COVID-19 condition prevalence at 28+ days from infection.Results
Fifty studies were included, and 41 were meta-analyzed. Global estimated pooled prevalence of post-COVID-19 condition was 0.43 (95% confidence interval [CI], .39–.46). Hospitalized and nonhospitalized patients had estimates of 0.54 (95% CI, .44–.63) and 0.34 (95% CI, .25–.46), respectively. Regional prevalence estimates were Asia (0.51; 95% CI, .37–.65), Europe (0.44; 95% CI, .32–.56), and United States of America (0.31; 95% CI, .21–.43). Global prevalence for 30, 60, 90, and 120 days after infection were estimated to be 0.37 (95% CI, .26–.49), 0.25 (95% CI, .15–.38), 0.32 (95% CI, .14–.57), and 0.49 (95% CI, .40–.59), respectively. Fatigue was the most common symptom reported with a prevalence of 0.23 (95% CI, .17–.30), followed by memory problems (0.14; 95% CI, .10–.19).Conclusions
This study finds post-COVID-19 condition prevalence is substantial; the health effects of COVID-19 seem to be prolonged and can exert stress on the healthcare system.
So what can we really say against this? Well, these kind of studies don’t really show that these symptoms are caused by COVID infection. Maybe they are caused by something else and are being thought of as being COVID’s long term effects. How can we tell these models apart? It’s simple: we both ask subjects about past COVID infections and measure this history of infection using a blood test. Then we add both to a model and see which predicts what kind of symptoms. This design was used by a French team studying a large sample:
- Matta, J., Wiernik, E., Robineau, O., Carrat, F., Touvier, M., Severi, G., … & Pastorino, B. (2022). Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA internal medicine, 182(1), 19-25.
Importance After an infection by SARS-CoV-2, many patients present with persistent physical symptoms that may impair their quality of life. Beliefs regarding the causes of these symptoms may influence their perception and promote maladaptive health behaviors.
Objective To examine the associations of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms (eg, fatigue, breathlessness, or impaired attention) in the general population during the COVID-19 pandemic.
Design, Setting, and Participants Participants in this cross-sectional analysis were 26 823 individuals from the French population-based CONSTANCES cohort, included between 2012 and 2019, who took part in the nested SAPRIS and SAPRIS-SERO surveys. Between May and November 2020, an enzyme-linked immunosorbent assay was used to detect anti–SARS-CoV-2 antibodies. Between December 2020 and January 2021, the participants reported whether they believed they had experienced COVID-19 infection and had physical symptoms during the previous 4 weeks that had persisted for at least 8 weeks. Participants who reported having an initial COVID-19 infection only after completing the serology test were excluded.
Main Outcomes and Measures Logistic regressions for each persistent symptom as the outcome were computed in models including both self-reported COVID-19 infection and serology test results and adjusting for age, sex, income, and educational level.
Results Of 35 852 volunteers invited to participate in the study, 26 823 (74.8%) with complete data for serologic testing and self-reported infection were included in the present study (mean [SD] age, 49.4 [12.9] years; 13 731 women [51.2%]). Self-reported infection was positively associated with persistent physical symptoms, with odds ratios ranging from 1.44 (95% CI, 1.08-1.90) to 16.61 (95% CI, 10.30-26.77) except for hearing impairment (odds ratio, 1.38; 95% CI, 0.76-2.51), joint pain (odds ratio, 1.32; 95% CI, 0.98-1.80) and sleep problems (odds ratio, 1.12; 95% CI, 0.87-1.44). A serology test result positive for SARS-COV-2 was positively associated only with persistent anosmia (odds ratio, 2.59; 95% CI, 1.57-4.28), even when restricting the analyses to participants who attributed their symptoms to COVID-19 infection. Further adjusting for self-rated health or depressive symptoms yielded similar results. There was no significant interaction between belief and serology test results.
Conclusions and Relevance The findings of this cross-sectional analysis of a large, population-based French cohort suggest that persistent physical symptoms after COVID-19 infection may be associated more with the belief in having been infected with SARS-CoV-2 than with having laboratory-confirmed COVID-19 infection. Further research in this area should consider underlying mechanisms that may not be specific to the SARS-CoV-2 virus. A medical evaluation of these patients may be needed to prevent symptoms due to another disease being erroneously attributed to “long COVID.”
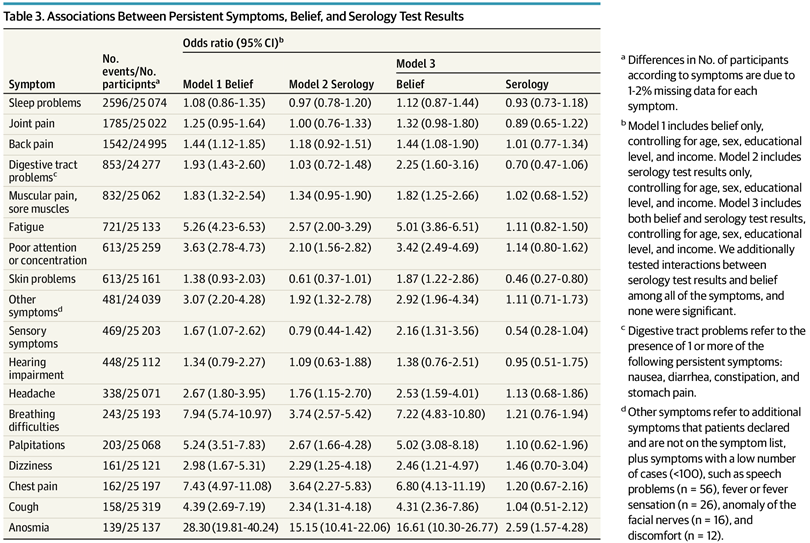
Their table of results:
These just show the crude counts/rates, so if you want to compare the belief vs. infection models, you need to compare the values across the different columns. We don’t need to do that because the authors also fit a model with both predictors and it looks like this:
Look at the Model 3 results in the rightmost columns. There are only 2 hits, i.e. p < .05. These are skin problems which were less common (OR 0.46) for people with COVID infection history, and anosmia (loss of smell) which was much more common (OR 2.59). The various things people usually claim, e.g. fatigue and concentration issues, were not different more than by chance with regards to infection history. BUT they were very different with regards to belief in COVID infections. In other words, the results of this huge study show that it was mainly belief in but not actual COVID infection that predicts long COVID symptoms. Basically, it indicates it is not real in the biological sense.
This study has been widely written about by skeptics of COVID orthodoxy. Noah Carl covered it, and so did Alex Berenson. Naturally, the ‘fact checkers’ have been trying hard to re-interpret the results and tell you they don’t mean what they plainly mean. But their spin aside, I wondered if there are any replications of this result. A single study may be a fluke or an error. So I looked over each of the 44 articles citing this study on Google Scholar, a kind of mini-systematic review. Here’s what I found.
First, there is an analytic replication of le French study, and now with only one author, who seems not to overlap with the original author list, plus it’s a preprint:
- Spiers, N. (2022). Reassessment of persistent symptoms, self-reported COVID-19 infection and SARS-CoV-2 serology in the SAPRIS-SERO cohort: identifying possible sub-syndromes of Long Covid. medRxiv.
Background Long Covid remains a relatively new phenomenon with emerging understanding. Estimated UK prevalence of Long Covid with three or more symptoms lasting for 12 weeks or more was 2.2% at the end of 2021. The population-based French SAPRIS-SERO cohort has novel information about the pattern of symptoms of Long Covid that has been obscured by controversy around the original paper.
Methods Secondary analysis was used to describe and re-interpret the frequencies of persistent symptoms by IgG seropositivity and self-reported Long Covid in the published report of the SAPRIS-SERO survey. Participants in the cross-sectional analysis were 26 823 individuals from the French population-based CONSTANCES cohort, included between 2012 and 2019, who took part in the nested SAPRIS and SAPRIS-SERO surveys. Between May and November 2020, the Euroimmun enzyme-linked immunosorbent assay was used to detect anti-SARS-CoV-2 antibodies. Surveyed online between December 2020 and January 2021, participants self-reported previous COVID-19 infection and physical symptoms during the previous four weeks that were new since March 2020, and had persisted for at least eight weeks.
Results There was similarity of prevalence over the majority of symptoms in those self-reporting COVID-19 infection, regardless of blood test result. Persistent symptoms significantly associated with self-reported COVID-19 infection and common in both groups include respiratory tract symptoms and a group of symptoms that might be related to chronic fatigue, malaise or postural issues. Seropositivity for IgG antibodies did not predict symptoms independently of self-reported Long Covid, except for anosmia.
Conclusions There may be three common sub-syndromes of Long Covid, one with persistent anosmia, another with other respiratory tract symptoms and a third, currently under researched, with symptoms relatable to chronic fatigue. Antibody tests are insufficient for case detection while Long Covid remains poorly understood.
The new version of the results:
Ignoring stuff above p > .01, we see that only anosmia was predicted by blood test result and most other stuff was predicted by belief in COVID. This is the same as the original publication, so we can trust they did the analyses correctly. That may not sound like much, but it is really something.
Second, I found a discussion of the Matta et al paper (le French study), which mentioned a replication by other authors. They said in their reply to Matta et al:
Dr Matta and colleagues 1 importantly recommend that those with persistent COVID-19 symptoms be adequately evaluated for conditions that may mimic post-acute sequelae of COVID-19. In arriving at this conclusion, they present results that replicate ours 2 : the only long-term symptom correlated with positive SARS-CoV-2 serology is anosmia. However, the evidence suggests that anosmia may be more helpful in assessing one’s likelihood of positive serology results than one’s history of COVID-19 infection. We have previously shown that those reporting persistent COVID-19 who are untested or who have tested negative have symptom time courses—with exception of change in smell and/or taste—that overlap with those who have had a positive result on reverse transcriptase–polymerase chain reaction (RT-PCR), antigen, or antibody test. We interpret this to mean that the clinical syndrome in people reporting persistent COVID-19 with positive, negative, or untested status, is the same. 2 The authors 1 make a key error in assuming that serology has good sensitivity for history of SARS- CoV-2 infection in all populations. Indeed, male sex and hospitalization were found to be predictors of greater antibody titers. 3 Additionally, though participants were asked to indicate whether they thought they had been infected since March 2020, 1 the serology results were obtained between May and November of 2020, suggesting that the time from the RT-PCR positive finding was significantly greater than the mean of 44 days in the study assessing sensitivity of the antibody assay. 4 As mentioned by the authors, antibodies wane with time and are less likely to be found in those with persistent symptoms.1 There is additionally no mention of statistical methods used to account for extremely large differences in between-group number of patients in the cohort, which can invalidate the results of the logistic regression analysis. Crucially, nonseroconversion after SARS-CoV-2 infection (that is, failure to test positive for antibodies at any time after a positive RT-PCR test result) occurs in an estimated 24% of cases. 5 Clearly, a negative serology finding is insufficient to rule out prior COVID-19 illness. Rigor must be applied to competing differential diagnoses, particularly those that are impossible to objectively dis- prove, such as some psychiatric conditions. In the absence of sufficient objective measures of prior infection, the patient’s clinical presentation should be the deciding factor when determining likelihood of prior infection. Further, a more important clinical question is why those with clinical syndromes consistent with persistent COVID-19 appear less likely to have sustained antibody responses.
[this is their entire letter, my emphasis]
I don’t really buy their criticism, and neither does Matta et al, and their reply to the reply is in the link above. Most interesting is their study they refer to, which is this one:
- Davis, H. E., Assaf, G. S., McCorkell, L., Wei, H., Low, R. J., Re’em, Y., … & Akrami, A. (2021). Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine, 38, 101019.
Background
A significant number of patients with COVID-19 experience prolonged symptoms, known as Long COVID. Few systematic studies have investigated this population, particularly in outpatient settings. Hence, relatively little is known about symptom makeup and severity, expected clinical course, impact on daily functioning, and return to baseline health.Methods
We conducted an online survey of people with suspected and confirmed COVID-19, distributed via COVID-19 support groups (e.g. Body Politic, Long COVID Support Group, Long Haul COVID Fighters) and social media (e.g. Twitter, Facebook). Data were collected from September 6, 2020 to November 25, 2020. We analyzed responses from 3762 participants with confirmed (diagnostic/antibody positive; 1020) or suspected (diagnostic/antibody negative or untested; 2742) COVID-19, from 56 countries, with illness lasting over 28 days and onset prior to June 2020. We estimated the prevalence of 203 symptoms in 10 organ systems and traced 66 symptoms over seven months. We measured the impact on life, work, and return to baseline health.Findings
For the majority of respondents (>91%), the time to recovery exceeded 35 weeks. During their illness, participants experienced an average of 55.9+/- 25.5 (mean+/-STD) symptoms, across an average of 9.1 organ systems. The most frequent symptoms after month 6 were fatigue, post-exertional malaise, and cognitive dysfunction. Symptoms varied in their prevalence over time, and we identified three symptom clusters, each with a characteristic temporal profile. 85.9% of participants (95% CI, 84.8% to 87.0%) experienced relapses, primarily triggered by exercise, physical or mental activity, and stress. 86.7% (85.6% to 92.5%) of unrecovered respondents were experiencing fatigue at the time of survey, compared to 44.7% (38.5% to 50.5%) of recovered respondents. 1700 respondents (45.2%) required a reduced work schedule compared to pre-illness, and an additional 839 (22.3%) were not working at the time of survey due to illness. Cognitive dysfunction or memory issues were common across all age groups (~88%). Except for loss of smell and taste, the prevalence and trajectory of all symptoms were similar between groups with confirmed and suspected COVID-19.Interpretation Patients with Long COVID report prolonged, multisystem involvement and significant disability. By seven months, many patients have not yet recovered (mainly from systemic and neurological/cognitive symptoms), have not returned to previous levels of work, and continue to experience significant symptom burden.
The relevant part of their article:
3.5 Symptoms by test result
Among respondents who received a diagnostic test (RT-PCR or antigen) for SARS-CoV-2 at any point during their illness, 1730 tested negative and 600 tested positive. The primary difference between these two groups was the time elapsed between symptom onset and testing, with a median of 6 days for those who tested positive and 43 days for those who tested negative (p < 0.001, Mann-Whitney U test) (Supplemental Figure S6). Symptoms were remarkably similar between the two groups. We compared symptom prevalence among positively and negatively tested respondents, stratified by test time. Out of 203 symptoms, 203 showed no statistically significant difference (p > 0.05; Fisher’s exact test, Bonferroni corrected). The loss of smell and taste were the only exceptions (loss of smell: 22.2% (negative) vs 60.8% (positive), p < 0.0001; 21.5% loss of taste: 21.5% (negative) vs. 54.9% (positive), p < 0.0001; Fisher’s exact test, Bonferroni corrected). In addition, 683 participants tested positive for SARS-CoV-2 antibodies (either IgG, IgM, or both).
Furthermore, respondents experienced similar variation in symptoms over time, despite differences in testing status. For 65 out of 66 symptoms, time courses overlapped substantially between participants with confirmed COVID-19 (n = 1020, positive RT-PCR, antigen, or antibody test at any point) and participants with no positive test result (n = 2742, Fig. 9). As above, change in smell/taste was the lone exception. Similar overlap was observed when separately comparing positively tested participants to negatively tested and untested participants (Supplemental Figures S7 and S8).
(I assume the double 203 vs. 203 is a typo and should be 202.) So it’s more or less a direct replication of le French study. Belief predicts symptoms much better than actual positive status, with the exception of the stuff one would expect: loss of smell and taste.
Third, there is another small replication study with even better methodology:
- Platteel, T. N., Koelmans, J. C., Cianci, D., Broers, N. J. H., de Bont, E. G. P. M., Cals, J. W. L., … & Verheij, T. J. M. (2022). Long-term prognosis of adults with moderate-severe SARS-CoV-2 lower respiratory tract infection managed in primary care: prospective cohort study. medRxiv.
Objectives To determine differences in health-related quality of life (HRQoL) and presence and duration of symptoms between adults with and without established SARS-CoV-2 moderately severe lower respiratory tract infection (LRTI) in the 12 months following their primary care visit.
Design Prospective cohort study
Setting 35 general practices in the provinces Noord-Brabant and Utrecht, the Netherlands.
Participants Individuals aged ≥18 years who presented to their general practitioner (GP) with a moderately severe LRTI during the first COVID-19 waive in The Netherlands (March-June 2021) underwent serology testing (participants, GPs and study personnel remained blinded for serology outcomes during study conduct) and completed baseline and follow-up questionnaires. Of the 315 participants who gave consent, 277 (88%) were suitable for inclusion in the analyses. Complete follow-up date was available in 97% of participants.
Main outcome measures 1) Scores of SF-36; physical component summary (PCS), mental component summary (MCS) and subscales. 2) Risk of any and individual persisting symptoms (of cough, dyspnea, chest pain, fatigue, brain fog, headache, and anosmia/ageusia) over time.
Results The change in SF-36 PSC (p=0.13), MCS (p=0.30), as well as subscale scores, over time did not differ between SARS-CoV-2 serology positive and negative participants after adjusting for sex, age, BMI, diabetes and chronic pulmonary conditions. The risk of any persisting symptom over time did not significantly differ between the groups (aHR 0.61, 95% CI 0.33-1.15), nor did the risk of individual symptoms.
Conclusions In the 12 months following their moderately severe LRTI, primary care patients with and without confirmed SARS-CoV-2 infection had a comparable HRQoL profile. Albeit a considerable proportion of patients reported persistent symptoms, there was no evidence of a difference in the course of symptoms over time between patients with and without confirmed SARS-CoV-2 infection.
This study looked at people who showed up to the doctor during COVID times with a flu-like disease (LRTI = lower respiratory tract infection). They were then tested but not told about their COVID result. Then they were followed up later and asked about long COVID symptoms. We then examine whether positive test result or belief in COVID infection predicts later symptoms better. A neat design that too allows us to see the difference between belief and disease. The authors describe the blinding:
To our knowledge, this is the first study in which both participants, as well as healthcare professionals and study personnel were blinded for the fact whether study participants had had a SARS-CoV-2 infection or not. This is important as blinding prevents different sorts of bias, like information and recall bias. Another strength of this study is the prospective design of our study and the inclusion of a SARS-CoV-2 serology negative control group of patients with a moderately severe LRTI and the standardized and detailed collection of important prognostic factors (confounders). This allowed us to determine the impact of LRTI etiology on patient’s HRQoL and persistence of symptoms.
Their outcomes were a mix (a factor score) of physical symptoms and mental symptoms:
Next, participants were asked to complete a questionnaire about demographics, presence and severity of symptoms (cough, dyspnea, chest pain, fatigue, brain fog, headache, anosmia/ageusia using a 0-4 Likert scale (0: absent, 1: a little, 2: moderate, 3: much and 4: very much) at time of the index consultation (recall) and at baseline, and their HRQoL two weeks prior to the index consultation (recall) and at baseline using the SF-36 questionnaire (15). This validated HRQoL questionnaire consists of a single item of health transition (HT) and a further 35 items which can be divided into 8 subscales: (1) physical function (PF), (2) limitations due to physical health problems (role physical, RP), (3) bodily pain (BP), (4) general health (GH), (5) vitality (VT), (6) social functioning (SF), (7) limitations due to emotional health problems (role-emotional, RE), and (8) mental health (MH). The scores of SF-36 between 0 and 100 were assigned to each domain, with higher scores indicating more favourable functional status. The eight subdomain scores were aggregated into two summary measures: physical component summary (PCS) scores and mental component summary (MCS) scores.
Results:
There is nothing to see for the physical ones:
Nor the mental ones.
What about that COVID reduced the brain size study?
I was thinking about what the strongest evidence of long COVID would be. The really, really best study would be that we infected people at random in a controlled trial. Seems insane? Not really, that’s what human challenge trials are for. But no one did this for COVID-19 as far as I know (but they should have!). What’s the next best study design? It would be some kind of study where we look at individuals over time and have before and after measures of the variables of interest. As getting hit by COVID is semi-random, this approach should not have much bias. There is a kind of study like this, and it got a lot of attention:
- Douaud, G., Lee, S., Alfaro-Almagro, F., Arthofer, C., Wang, C., McCarthy, P., … & Smith, S. M. (2022). SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature, 604(7907), 697-707.
There is strong evidence of brain-related abnormalities in COVID-191,2,3,4,5,6,7,8,9,10,11,12,13. However, it remains unknown whether the impact of SARS-CoV-2 infection can be detected in milder cases, and whether this can reveal possible mechanisms contributing to brain pathology. Here we investigated brain changes in 785 participants of UK Biobank (aged 51–81 years) who were imaged twice using magnetic resonance imaging, including 401 cases who tested positive for infection with SARS-CoV-2 between their two scans—with 141 days on average separating their diagnosis and the second scan—as well as 384 controls. The availability of pre-infection imaging data reduces the likelihood of pre-existing risk factors being misinterpreted as disease effects. We identified significant longitudinal effects when comparing the two groups, including (1) a greater reduction in grey matter thickness and tissue contrast in the orbitofrontal cortex and parahippocampal gyrus; (2) greater changes in markers of tissue damage in regions that are functionally connected to the primary olfactory cortex; and (3) a greater reduction in global brain size in the SARS-CoV-2 cases. The participants who were infected with SARS-CoV-2 also showed on average a greater cognitive decline between the two time points. Importantly, these imaging and cognitive longitudinal effects were still observed after excluding the 15 patients who had been hospitalised. These mainly limbic brain imaging results may be the in vivo hallmarks of a degenerative spread of the disease through olfactory pathways, of neuroinflammatory events, or of the loss of sensory input due to anosmia. Whether this deleterious effect can be partially reversed, or whether these effects will persist in the long term, remains to be investigated with additional follow-up.
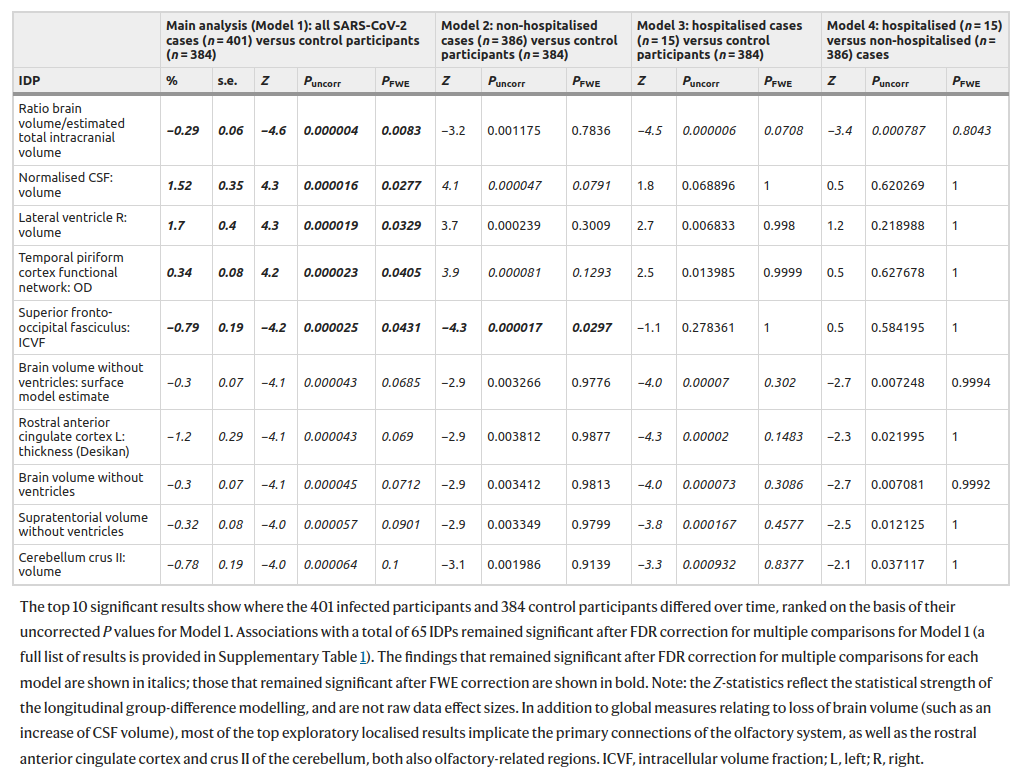
It sure sounds good! UK Biobank is an awesome resource. They use pre-post design, i.e., with self-control measures. And it says “a greater reduction in global brain size in the SARS-CoV-2 cases”. So I read the study carefully. My finding is that I can’t really tell what exactly they were doing in this analysis! They talk a lot about this longitudinal aspect, but then they keep talking about cases and controls in their comparisons. There are no cases and controls in a within-person design, so these appear to be just the matched control results. Here’s their big table of exploratory results:
They did 2047 tests for each model. I am just going to look at the first model in the left column. Of the 2047 tests, 5 are p < .05 when adjusted for multiple testing (“fwe”). Of these, 4 are p > .01, and one single result is p = .0083. This evidence is not very impressive, is it? Next, notice that the effect sizes are tiny. The most important looking variable is brain volume ratio of the intracranial volume, i.e., how much space the brain takes up of the inner cranium. The change here was -0.29%. Less than 1 in 300 part smaller brain volume. What they are saying is that among the relatively old people in this sample who got COVID, their brain sizes shrunk by a statistically reliable, but tiny amount. I interpret this in line with old people in general failing to fully recover after serious illness, but most of them recovering most of the way back to their pre-sickness trend-line. This result is nothing to worry about.
Then there’s the cognitive decline, shown above. The UKBB intelligence tests kinda suck, but there’s this one trail making test, and it shows a scary looking gap. Unfortunately, the results are presented in % change instead of Cohen’s d, so we can’t really tell how bad this is. I couldn’t find any more information about these results, and only 2 of the 10 cognitive score results are mentioned. What did the others show? The paper is silent as far as I can tell. In any case, even if we take the most extreme result from the trail B test, it basically shows there is no long COVID for those below age 60. Most people loudly claiming long COVID on social media are much younger than that, so even this extreme result provides zero support for them.
Going back to the abstract and reading more carefully. It seems they didn’t actually do a within person study! They just used the pre-COVID scans to validate that their case-control split didn’t show detectable bias. What a wasted opportunity!
I couldn’t find any more test vs. belief studies. If you can find one, please post it in the comments! Or email me if you want to be anon (emilowk@proton.me). Alex Berenson mentions another study in this paywalled post. Can someone post the link to it?
Overall, the results are consistent. We have two large studies, and one smaller study with even better methodology. We additionally have a reanalysis of the first large study. The results are consistent across the publications and datasets. The conclusion then seems fairly certain that long COVID is mostly not caused by COVID per se, but must have another origin. The obvious candidate is psychosomatics AKA it’s all in your head. I wouldn’t say these results prove it is 100% not real, but at least, we have to lean heavily in that direction, let’s say 90% not real. In line with this copycat model, there’s several studies showing that long COVID is related to mental illness, including pre-infection diagnoses (i.e. reverse causation not possible), and the other proxy for hypochondriacism, which is being a woman. E.g. this study found:
A total of 377 patients were enrolled in the study. The median time from symtpom onset to virological clerance was 44 (37–53) days. A diagnosis of long COVID syndrome was made in 260/377 (69%) patients. The most common reported symptoms were fatigue (149/377, 39.5%), exertional dyspnoea (109/377, 28.9%), musculoskeletal pain (80/377, 21.2%) and “brain fog” (76/377, 20.2%). Anxiety symptoms were ascertained in 71/377 (18.8%) individuals, whereas 40/377 (10.6%) patients presented symptoms of depression. Post-traumatic stress disorder (defined by a pathological IES-R score) was diagnosed in one-third of patients (85/275, 31%). Female gender was independently associated with long COVID syndrome at multivariable analysis (AOR 3.3 vs. males, 95% CI 1.8–6.2, p < 0.0001). Advanced age (adjusted (A)OR 1.03 for 10 years older, 95% CI 1.01–1.05, p 0.01) and active smoking (AOR 0.19 for former smokers vs. active smokers, 95% CI 0.06–0.62, p 0.002) were also associated with a higher risk of long COVID, while no association was found between severity of disease and long COVID (AOR 0.67 for continuous positive airway pressure (CPAP)/non-invasive mechanical ventilation (NIMV)/orotracheal intubation (OTI) vs. no 02 therapy, 95% CI 0.29–1.55, p 0.85).
And there’s a replication here:
Note that female and anxiety disorder lights up despite the smallish sample size. I didn’t look carefully for more of these studies as I don’t think anyone will seriously doubt the findings.
To be clear: these results don’t show that long-COVIDers are faking it. Fakers are deliberately misleading people into thinking they have symptoms they don’t really have. I am saying here that the results show it is substantially and mostly not real, as in, not caused by the disease caused by the virus, but by people internalizing a set of symptoms which is related to the disease and which they also learn from the media and from each other. A typical case of mass sociogenic illness, like the multiple personality disorder craze in the 1980s. For a recent, amusing example, check out the tic case from Germany:
- Müller-Vahl, K. R., Pisarenko, A., Jakubovski, E., & Fremer, C. (2022). Stop that! It’s not Tourette’s but a new type of mass sociogenic illness.
We report the first outbreak of a new type of mass sociogenic illness that in contrast to all previously reported episodes is spread solely via social media. Accordingly, we suggest the more specific term ‘mass social media-induced illness’.
In Germany, the current outbreak of mass social media-induced illness is initiated by a ‘virtual’ index case, who is the second most successful YouTube creator in Germany and enjoys enormous popularity among young people. Affected teenagers present with similar or identical functional ‘Tourette-like’ behaviours, which can be clearly differentiated from tics in Tourette syndrome.
Functional ‘Tourette-like’ symptoms can be regarded as the ‘modern’ form of the well-known motor variant of mass sociogenic illness. Moreover, they can be viewed as the 21st century expression of a culture-bound stress reaction of our post-modern society emphasizing the uniqueness of individuals and valuing their alleged exceptionality, thus promoting attention-seeking behaviours and aggravating the permanent identity crisis of modern man. We wish to raise awareness of the current global Tourette-like mass social media-induced illness outbreak. A large number of young people across different countries are affected, with considerable impact on health care systems and society as a whole, since spread via social media is no longer restricted to specific locations such as local communities or school environments spread via social media is no longer restricted to specific locations such as schools or towns.